Abstract
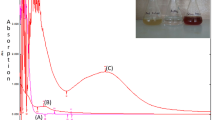
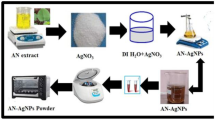
The plant extracts were applied for synthesis of Ag-nanoparticles and expected such biological processes benefit from advantages like eco-friendly, cost-effective, and safe for human use. Accordingly, polyphenol compounds and antioxidant properties of this understudy plant extract was initially qualified and quantified by GC-Mass and subsequently its ability for silver nanoparticle (AgNP) biosynthesis was examined. Consequently, under study (ethanol) extract was exposed to various content of AgNO3 solution over 0, 2.5, 5, 7.5, 10 and 15 mM for 24, 48, 72 and 96 h to search best operational conditions for AgNPs production, while sampling were done every 24 h monitoring initial absorption with UV–Vis spectroscopy. The peak with center at 460 nm emerged from surface plasmon resonance of AgNPs which is good indication of successful formation of AgNPs. The nanoparticles were characterized by DLS, SEM, TEM, XRD, and FTIR. Which reveal its spherical, shape and average diameter of 32 nm. Antioxidant activity tests showed that the half-maximal inhibitory concentration (IC50) in the C. tuberculata extract was 4.722 mg/ml. Antimicrobial activity of the AgNPs was tested by measurement of inhibition zone around discs modified with AgNPs in cultures of S. aureus, B. cereus, P. aeruginosa and E. coli.







Similar content being viewed by others
References
O.V. Salata, Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2(1), 3 (2004)
I. Sondi, B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275(1), 177–182 (2004)
P.S. Basavarajappa, S.B. Patil, N. Ganganagappa, K.R. Reddy, A.V. Raghu, C.V. Reddy, Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy. 45(13), 7764–7778 (2020)
R. Cai, Y. Du, D. Yang, G. Jia, B. Zhu, B. Chen, W. Chen, Free-standing 2D nanorafts by assembly of 1D nanorods for biomolecule sensing. Nanoscale. 11(25), 12169–12176 (2019)
R. Gao, X. Qin, T. Fan, R. Xu, H. Wu, Z. Wang, W. Cai, Influence of IrO2 addition on magnetoelectric properties of Ni 0.5 Zn 0.5 Fe2O4/Ba 0.8 Sr 0.2 TiO3 composite ceramics. J. Mater. Sci. Mater. Electron. 31(3), 2436–2445 (2020)
B. Khodashenas, H.R. Ghorbani, Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 12(8), 1823–1838 (2019)
C.V. Reddy, I.N. Reddy, K. Ravindranadh, K.R. Reddy, N.P. Shetti, D. Kim, T.M. Aminabhavi, Copper-doped ZrO2 nanoparticles as high-performance catalysts for efficient removal of toxic organic pollutants and stable solar water oxidation. J. Environ. Manage. 260, 110088 (2020)
N.P. Shetti, S.D. Bukkitgar, K.R. Reddy, C.V. Reddy, T.M. Aminabhavi, Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Coll. Surf B Biointerfaces (2019). https://doi.org/10.1016/j.colsurfb.2019.03.013
O. Choi, K.K. Deng, N.-J. Kim, L. Ross Jr., R.Y. Surampalli, Z. Hu, The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 42(12), 3066–3074 (2008)
A.M. Fayaz, K. Balaji, P. Kalaichelvan, R. Venkatesan, Fungal based synthesis of silver nanoparticles—an effect of temperature on the size of particles. Coll. Surf. B. Biointerfaces. 74(1), 123–126 (2009)
S. Ghojavand, M. Madani, J. Karimi, Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of Felty germander. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01449-1
N. Pugazhenthiran, S. Anandan, G. Kathiravan, N.K.U. Prakash, S. Crawford, M. Ashokkumar, Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanopart. Res. 11(7), 1811 (2009)
J. **e, J.Y. Lee, D.I. Wang, Y.P. Ting, Silver nanoplates: from biological to biomimetic synthesis. ACS Nano 1(5), 429–439 (2007)
A.K. Singh, R. Tiwari, V. Kumar, P. Singh, S.R. Khadim, A. Tiwari, R. Asthana, Photo-induced biosynthesis of silver nanoparticles from aqueous extract of Dunaliella salina and their anticancer potential. J. Photochem. Photobiol. B. 166, 202–211 (2017)
G. Mahendran, B.R. Kumari, Biological activities of silver nanoparticles from Nothapodytes nimmoniana (Graham) Mabb. fruit extracts. Food Sci. Hum. Wellness 5(4), 207–218 (2016)
M.I. Rashid, L.H. Mujawar, Z.A. Rehan, H. Qari, J. Zeb, T. Almeelbi, I.M. Ismail, One-step synthesis of silver nanoparticles using Phoenix dactylifera leaves extract and their enhanced bactericidal activity. J. Mol. Liq. 223, 1114–1122 (2016)
K. Jadhav, D. Dhamecha, D. Bhattacharya, M. Patil, Green and ecofriendly synthesis of silver nanoparticles: characterization, biocompatibility studies and gel formulation for treatment of infections in burns. J. Photochem. Photobiol. B 155, 109–115 (2016)
E. Abdel-Sattar, N.G. Shehab, C. Ichino, H. Kiyohara, A. Ishiyama, K. Otoguro, H. Yamada, Antitrypanosomal activity of some pregnane glycosides isolated from Caralluma species. Phytomed. 16(6–7), 659–664 (2009)
M. Soltanipour, Medicinal plants of the geno protected area. Pajouhesh-Va-Sazandegi Fall 18, 27–37 (2005)
N. Ahmad, S. Sharma, M.K. Alam, V. Singh, S. Shamsi, B. Mehta, A. Fatma, Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf. B. Biointerfaces 81(1), 81–86 (2010)
H.C. Dutt, S. Singh, B. Avula, I.A. Khan, Y.S. Bedi, Pharmacological review of Caralluma R. Br. with special reference to appetite suppression and anti-obesity. J. Med. Food. 15(2), 108–119 (2012)
A. Pothig, S. Ahmed, H.C. Winther-Larsen, S. Guan, P.J. Altmann, J. Kudermann, O.A. Hogmoen Astrand, Antimicrobial activity and cytotoxicity of Ag (I) and Au (I) pillarplexes. Front. Chem. 6, 584 (2018)
E. Rodriguez-Leon, R. Iniguez-Palomares, R.E. Navarro, R. Herrera-Urbina, J. Tanori, C. Iniguez-Palomares, A. Maldonado, Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 8(1), 318 (2013)
J. Poodineh, A. Khazaei Feizabad, A. Nakhaee, Antioxidant Activities of Caralluma tuberculata on Streptozotocin-Induced Diabetic Rats. Drug Dev. Res. 76(1), 40–47 (2015)
L. Sun, J. Zhang, X. Lu, L. Zhang, Y. Zhang, Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 49(10), 2689–2696 (2011)
S.-Y. Mok, M.J. Choi, J. Kim, E.J. Cho, S. Lee, Antioxidant activity of the methanolic extract of the newly generated vegetable, baemuchae (xBrassicoraphanus). Food Chem. Toxicol. 50(3–4), 848–853 (2012)
A.H. El-Ghorab, M. Nauman, F.M. Anjum, S. Hussain, M. Nadeem, A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 58(14), 8231–8237 (2010)
H.-L. Siow, C.-Y. Gan, Extraction, identification, and structure–activity relationship of antioxidative and α-amylase inhibitory peptides from cumin seeds (Cuminum cyminum). J. Funct. Foods. 22, 1–12 (2016)
A.D. Dwivedi, K. Gopal, Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A. Physicochem. Eng. Asp. 369(1–3), 27–33 (2010)
D. Inbakandan, R. Venkatesan, S.A. Khan, Biosynthesis of gold nanoparticles utilizing marine sponge Acanthella elongata (Dendy, 1905). Colloids Surf. B. Biointerfaces. 81(2), 634–639 (2010)
D. Elumalai, P. Kaleena, K. Ashok, A. Suresh, M. Hemavathi, Green synthesis of silver nanoparticle using Achyranthes aspera and its larvicidal activity against three major mosquito vectors. Eng. Agric. Environ. Food. 9(1), 1–8 (2016)
S. Soman, J. Ray, Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) Mill: characterization and assessment of antibacterial activity. J. Photochem. Photobiol. B. 163, 391–402 (2016)
J. Karimi, S. Mohsenzadeh, Plant synthesis of silver nanoparticles by Achillea wilhelmsii Pharmaceutical plant. Razi J. Med. Sci. 20(111), 64–69 (2013)
S.S. Shankar, A. Rai, A. Ahmad, M. Sastry, Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 275(2), 496–502 (2004)
S. Khorrami, A. Zarrabi, M. Khaleghi, M. Danaei, M. Mozafari, Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 13, 8013 (2018)
L. Zang, J. Qiu, X. Wu, W. Zhang, E. Sakai, Y. Wei, Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res. 53(9), 3448–3454 (2014)
K. Kathiresan, S. Manivannan, M. Nabeel, B. Dhivya, Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Coll. Surf. B. Biointerfaces. 71(1), 133–137 (2009)
G. Singhal, R. Bhavesh, K. Kasariya, A.R. Sharma, R.P. Singh, Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart. Res. 13(7), 2981–2988 (2011)
M. Yilmaz, H. Turkdemir, M.A. Kilic, E. Bayram, A. Cicek, A. Mete, B. Ulug, Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana. Mater. Chem. Phys. 130(3), 1195–1202 (2011)
F.A. Cunha, K.R. Maia, E.J.J. Mallman, M.D.C.D.S. Cunha, A.A.M. Maciel, I.P.D. Souza, P.B.A. Fechine, Silver nanoparticles-disk diffusion test against Escherichia coli isolates. Rev. Inst. Med. Trop. Sao Paulo (2016). https://doi.org/10.1590/S1678-9946201658073
S.-H. Kim, H.-S. Lee, D.-S. Ryu, S.-J. Choi, D.-S. Lee, Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 39(1), 77–85 (2011)
D. Paredes, C. Ortiz, R. Torres, Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157: H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Nanomed. 9, 1717 (2014)
S. Pal, Y.K. Tak, J.M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73(6), 1712–1720 (2007)
Acknowledgments
This research was supported by Behbahan Khatam Alanbia University of Technology. We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zarei, Z., Razmjoue, D. & Karimi, J. Green Synthesis of Silver Nanoparticles from Caralluma tuberculata Extract and its Antibacterial Activity. J Inorg Organomet Polym 30, 4606–4614 (2020). https://doi.org/10.1007/s10904-020-01586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01586-7