Abstract
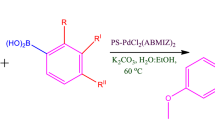
A new polymer-anchored Pd(II) complex has been synthesized, characterized and its catalytic activity was investigated for the Suzuki cross-coupling reaction between aryl halides and arylboronic acid in the presence of K2CO3 as a base, for Heck olefination of aryl halides with alkenes, and for cyanation reaction of aryl iodides with K4Fe(CN)6 in presence of Et3N as base. The key features of the catalyst include rapid reactions with excellent conversion without the use of phosphine ligands and total stability under the reactions conditions. The catalyst was recovered by simple filtration and reused five-times without significant loss of catalytic activity.








Similar content being viewed by others
References
N. Miyaura, A. Suzuki, Chem. Rev. 95, 2457 (1995)
A. Suzuki, J. Organomet. Chem. 576, 147 (1999)
R.F. Heck, J.P. Nolley, J. Org. Chem. 37, 2320 (1972)
P. Baumeister, W.M.K. Oertle, G. Seifert, H. Steiner, Chimia 51, 144 (1997)
N. Gurbuz, S. Vural, S. Yasar, I. Ozdemir, T. Seckin, J. Inorg. Orgnomet. Polym. doi:10.1007/s10904-009-9315-3
R.L. Elsenbauer, L.W. Schacklett, in Handbook of Conducting Polymer, ed. by T.A. Skotheim, vol 1, chap 7 (Marcel-Dekker, New work, 1986)
G. Solladle, G. Gottarelli, Tetrahedron 43, 1425 (1987)
J.P. Wolfe, R.A. Singer, B.H. Yang, S.L. Buchwald, J. Am. Chem. Soc. 121, 9550 (1999)
A.F. Littke, C. Dai, G.C. Fu, J. Am. Chem. Soc. 122, 4020 (2000)
I.P. Beletskaya, A.V. Cheprakov, Chem. Rev. 100, 3009 (2000)
D. Song, W.B. Yi, J. Mol. Catal. A: Chem. 280, 20 (2008)
K.M. Wu, C.A. Huang, K.F. Peng, C.T. Chen, Tetrahedron 61, 9679 (2005)
K. Sarkar, M. Nandi, M. Islam, M. Mubarak, A. Bhaumik, Appl. Catal. A: Gen. 352, 81 (2009)
A. Corma, H. Garcia, A. Leyva, J. Mol. Catal. A: Chem. 230, 97 (2005)
S. Paul, J.H. Clark, Green Chem. 5, 635 (2003)
V. Polshettiwar, C. Len, A. Fihri, Coord. Chem. Rev. 253, 2599 (2009)
N.T.S. Phan, D.H. Brown, P. Styring, Tetrahedron Lett. 45, 7915 (2004)
V. Polshettiwar, R.S. Varma, Tetrahedron 64, 4637 (2008)
S. Mukherjee, S. Samanta, B.C. Roy, A. Bhaumik, Appl. Catal. A: Gen. 301, 79 (2006)
K. Shimizu, S. Koizumi, T. Hatamachi, H. Yoshida, S. Komai, T. Kodama, Y. Kitayama, J. Catal. 228, 141 (2004)
H. Bulut, L. Artoka, S. Yilmaz, Tetrahedron Lett. 44, 289 (2003)
F.Y. Tsai, C.L. Wu, C.Y. Mou, M.C. Chao, H.P. Linc, S.T. Liua, Tetrahedron Lett. 45, 7503 (2004)
A. Papp, G. Galbacs, A. Molnar, Tetrahedron Lett. 46, 7725 (2005)
K. Okumura, K. Nota, K. Yoshida, M. Niwa, J. Catal. 231, 245 (2005)
J.W. Kim, J.H. Kim, D.H. Lee, Y.S. Lee, Tetrahedron Lett. 47, 4745 (2006)
C.A. Lin, F.T. Luo, Tetrahedron Lett. 44, 7565 (2003)
M. Islam, P. Mondal, S. Mondal, S. Mukherjee, A.S. Roy, M. Mubarak, M. Paul, J. Inorg. Orgnomet. Polym. doi:10.1007/S10904-009-9310-8
O. Grossmann, D. Gelman, Org. Lett. 8, 1189 (2006)
T. Schareina, A. Zapf, M. Beller, Chem. Commun. 1388 (2004)
T. Schareina, A. Zapf, W. Mägerlein, N. Müller, M. Beller, Tetrahedron Lett. 48, 1087 (2007)
J. Zanon, A. Klapars, S.L. Buchwald, J. Am. Chem. Soc. 125, 2890 (2003)
A. Littke, M. Soumeillant, R.F. Kaltenbach, R.J. Cherney, C.M. Tarby, S. Kiau, Org. Lett. 9, 1711 (2007)
A.I. Vogel, Text Book of Practical Organic Chemistry (Quantitative Analysis), 5th edn. (Longman, London, 1989)
R.B. King, E.M. Sweet, J. Org. Chem. 44, 385 (1979)
S.M. Islam, A. Bose, B.K. Palit, C.R. Saha, J. Catal. 173, 268 (1998)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic and Bioinorganic Chemistry, 6th edn. (John Wiley and Sons, New York, 2009), p. 65
J.R. Durig, R. Layton, D.W. Sink, B.R. Mitchell, Pectrochim. Acta 21, 1367 (1965)
S. Jayasree, A. Seayad, R.V. Chaudhari, Org. Lett. 2, 203 (2000)
S.I. Mostafa, Transition Metal Chem. 32, 769 (2007)
B. Karimi, D. Enders, Org. Lett. 8, 1237 (2006)
J. Rebek, D. Brown, S. Zimmerman, J. Am. Chem. Soc. 97, 454 (1975)
J. Rebek, F. Gavina, J. Am. Chem. Soc. 69, 7112 (1974)
Acknowledgements
We thank the Indian Association for the Cultivation of Science for providing the instrumental support. We acknowledge the Department of Science and Technology (DST), Council of Scientific and Industrial Research (CSIR) and the University Grant Commission (UGC), New Delhi, India for funding. We also thank the DST and UGC, New Delhi, India for providing instrumental support under the FIST and SAP program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, S.M., Mondal, P., Tuhina, K. et al. A Reusable Polymer-Anchored Palladium(II) Schiff Base Complex Catalyst for the Suzuki Cross-Coupling, Heck and Cyanation Reactions. J Inorg Organomet Polym 20, 264–277 (2010). https://doi.org/10.1007/s10904-010-9352-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-010-9352-y