Abstract
The canola flower midge, Contarinia brassicola Sinclair (Diptera: Cecidomyiidae), is a newly-described species that induces galls on canola, Brassica napus Linnaeus and Brassica rapa Linnaeus (Brassicaceae). Identification of the sex pheromone of C. brassicola is essential to develo** monitoring tools to elucidate the geographic range and hosts of this new pest, and the extent to which it threatens the $30 billion Canadian canola industry. The aim of this study was to identify and synthesize the female-produced sex pheromone of C. brassicola and demonstrate its effectiveness in attracting males to traps in the field. Two peaks were identified through GC-EAG analysis of female-produced volatiles which elicited electrophysiological responses in male antennae. These peaks were initially characterized through GC–MS and synthesis as 2,7-diacetoxynonane (major component) and 2-acetoxynonane (minor component), and the racemic compounds elicited EAG responses in male antennae. All four stereoisomers of 2,7-diacetoxynonane were synthesized and the naturally-produced compound was shown to be primarily the (2R,7S)-isomer by analysis on an enantioselective GC column, with a small amount of (2R,7R)-2,7-diacetoxynonane also present. The configuration of the minor component could not be determined because of the small amount present, but this was assumed to be (2R)-2-acetoxynonane by comparison with the configuration of the other two components. In field trials, none of the four stereoisomers of 2,7-diacetoxynonane, presented individually or as a racemic mixture, was attractive to male C. brassicola. However, dispensers loaded with a 10 µg:1 µg blend of (2R,7S)- and (2R,7R)-2,7-diacetoxynonane caught large numbers of male C. brassicola and significantly more than other blends tested. The addition of 0.5 µg of (2R)-2-acetoxynonane to this blend further increased the number of males caught. In future work, we will seek to identify the optimum trap** protocol for the application of the pheromone in monitoring and surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The canola flower midge, Contarinia brassicola Sinclair (Diptera: Cecidomyiidae), induces galls on canola, Brassica napus Linnaeus and Brassica rapa Linnaeus (Brassicaceae) (Mori et al. 2019). The galls were first discovered in the province of Saskatchewan in 2012 and originally attributed to the swede midge, Contarinia nasturtii (Kieffer) (Diptera: Cecidomyiidae), the only other species of cecidomyiid known to infest canola in North America (Mori et al. 2019). However, adults of the two species exhibited differences in the morphology of the wings, antennae, and genitalia, as well as the shape of flower galls produced. Results of phylogenetic analyses also supported C. brassicola as a distinct species within the genus Contarinia (Mori et al. 2019). Contarinia brassicola appears to be the main species of Contarinia on canola across the Canadian prairies, where C. nasturtii has not been detected since 2007 (Mori et al. 2019; Vankosky et al. 2022). The origin of C. brassicola, and its importance as a pest species threatening the $30 billion Canadian canola industry (Canola Council of Canada 2021), remain to be determined.
Contarinia brassicola adults begin to emerge in June and July on the Canadian prairies, which coincides with bud formation and early flowering in canola (Mori et al. 2019; Vankosky et al. 2022). Females lay eggs on develo** canola buds, and, after hatching, larvae feed cryptically within the flower bud resulting in gall formation. These galls prevent pod formation and result in yield loss. Mature larvae leave the galled flowers and form cocoons in the soil. A portion of the larvae pupate and emerge as a second generation, while others appear to undergo diapause for emergence the following year (Campbell et al. 2020; Vankosky et al. 2022). To date, C. brassicola has only been found in North America (Campbell et al. 2020; Mori et al. 2019).
Adult females of at least 19 species of Cecidomyiidae have been demonstrated to produce sex pheromones attractive to males (Hall et al. 2012; Xu et al. 2012).
Traps were white Jackson traps (Distributions Solida, Saint-Ferréol-les-Neiges, Québec, Canada) and pheromone dispensers were closed polyethylene vials (26 mm × 8 mm × 1.5 mm thick; Just Plastics Ltd., London, UK). The latter were found to give more sustained release than rubber septa with compounds of similar molecular weight to those tested here (Rowley et al. 2017). The vials were impregnated by applying the pheromone in hexane solution containing 10% 2,6-di-tert-butyl-4-methylphenol (BHT) as antioxidant (100 µl), allowing the solvent to evaporate fully and then cap** the vial. Lures were prepared in the UK, stored in heat-sealed aluminium foil bags, and shipped to Canada where they were kept in a refrigerator (4 °C) before use.
In Experiment 1, catches of C. brassicola were compared in traps baited with each of the four isomers of 2,7-diacetoxynonane individually (10 µg), the racemic mixture of the four isomers (40 µg) and an unbaited trap. After one week, traps, inserts, and lures were removed and replaced, and treatment position randomized within blocks. Numbers of male and female C. brassicola on each trap were counted each week, with the experiment repeated over two weeks from 28 June to 12 July 2018.
In Experiment 2, the effects of adding possible minor components (2R,7R)-diacetoxynonane and (2R)-2-acetoxynonane to the proposed major pheromone component, (2R,7S)-diacetoxynonane were investigated. Numbers of C. brassicola caught were compared between traps baited with dispensers loaded with one of five treatments: solvent only control; 10 µg (2R,7S)-2,7-diacetoxynonane; 10 µg (2R,7S)-2,7-diacetoxynonane plus 1 µg, 5 µg or 10 µg (2R,7R)-2,7-diacetoxynonane; and 10 µg (2R,7S)-2,7-diacetoxynonane plus 10 µg (2R,7R)-2,7-diacetoxynonane and 1 µg (2R)-2-acetoxynonane. The experiment ran from 27 June to 7 August 2019 and lures were changed once on 11 July. Traps were monitored and sticky liners replaced at ca. 1-week intervals, and treatment positions randomized. Numbers of male and female C. brassicola on each trap were counted each week.
In Experiment 3, the ratio of pheromone components was further refined, based on the treatments which caught the most male C. brassicola in Experiment 2. Traps were baited with dispensers loaded with one of seven treatments: solvent only control; 10 µg (2R,7S)-2,7-diacetoxynonane plus 0.1 µg, 0.5 µg, 1 µg, 2 µg or 5 µg (2R,7R)-2,7-diacetoxynonane; and 10 µg (2R,7S)-2,7-diacetoxynonane plus 1 µg (2R,7R)-2,7-diacetoxynonane and 0.5 µg (2R)-2-acetoxynonane. The experiment ran from 8–28 August 2019. Traps were monitored and sticky liners replaced at ca. 1-week intervals, and treatment positions randomized. Numbers of male and female C. brassicola on each trap were counted each week.
Statistical Analyses
General linear mixed models were used to compare numbers of male C. brassicola caught in the different treatments (Bates et al. 2015). Numbers of males caught per trap per week were transformed to log(n + 1) and entered as the dependent variable and treatment entered as an independent factor (Experiment 1: six levels, Experiment 2: seven levels). Trap week and field site were entered as random factors. Significance of the treatment term within each model was assessed through χ2 tests of changes in residual deviance following deletion from the model (Pinheiro and Bates 2000). The significance of differences (P < 0.05) between catches with different lures in each experiment were assessed using Tukey’s post-hoc tests on estimated marginal means (Lenth 2020). All data analyses were performed in R 4.0.2 (R Core Team 2020).
Results
Pheromone Identification
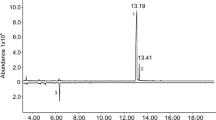
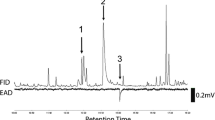
In GC-EAG analyses of volatile collections from virgin female C. brassicola on a polar DBWax GC column with antennae of virgin male C. brassicola, two consistent EAG responses were observed (Fig. 2). These had retention indices (RI) of 1443 and 1968, with the latter response larger than the former, corresponding to an apparently larger peak in the FID chromatogram (Fig. 2). The compounds responsible for these responses were assumed to be minor and major components, respectively, of the female-produced sex pheromone of C. brassicola.
GC-EAG analyses of volatile collection from virgin female Contarinia brassicola with male C. brassicola EAG preparation on polar GC column showing EAG responses (*) to compounds proposed as major (1) and minor (2) pheromone components; lower chromatogram is expansion of upper; in each chromatogram lower trace is FID, upper traces EAG responses from three different males
In analyses of the volatile collections by GC–MS on a similar polar DBWax GC column, a female-specific peak was observed at RI 1968 (Fig. 3) for the major pheromone component. This compound had the mass spectrum shown in Fig. 4, which was remarkably similar to those reported for 2,7-diacetoxyundecane, major component of the sex pheromone of the pear midge, C. pyrivora (Riley) (Amarawardana 2009; Hall et al. 2012), 2,7-dibutyroxynonane, sex pheromone of the orange wheat blossom midge, Sitodiplosis mosellana (Géhin) (Gries et al. 2000; Hooper et al. 2007), and 2,7-diacetoxytridecane, sex pheromone of the aphidophagous gall midge, Aphidoletes aphidimyza (Rondi) (Choi et al. 2004).
The RI 1968 indicated the compound had two fewer carbon atoms than 2,7-diacetoxyundecane (RI 2167; Amarawardana 2009), and the 2,7-diacetoxynonane structure was consistent with the mass spectrum (Fig. 4). Fragmentation ions at m/z 43 and 61 suggested the presence of acetate group(s). The ion at m/z 126 corresponded to the loss of two acetoxy groups from a just-detectable molecular ion at m/z 244, and that at m/z 124 to the loss of two acetic acid molecules from the molecular ion. Loss of an ethyl group from the latter would give the strong ion at m/z 95, providing evidence for one of the acetate groups at C-7. Loss of a methyl group from m/z 124 would give the ion at m/z 109 confirming the position of the other acetate group at C-2, in line with all midge pheromones reported to date which have an oxygen functionality at C-2 (Hall et al. 2012; Xu et al. 9). Traps baited with lures loaded with 10 µg (2R,7S)-2,7-diacetoxynonane plus 1.0 µg (2R,7R)-2,7-diacetoxynonane caught the most male C. brassicola, and significantly more males than those baited with lures loaded with 10 µg (2R,7S)-2,7-diacetoxynonane plus 5 µg (2R,7R)-2,7-diacetoxynonane or 10 µg (2R,7S)-2,7-diacetoxynonane plus 10 µg (2R,7R)-2,7-diacetoxynonane plus 1 µg (2R)-2-acetoxynonane (Tukey’s test. P < 0.05). Traps baited with the two latter treatments caught significantly more males than those baited with 10 µg (2R,7S)-2,7-diacetoxynonane alone, 10 µg (2R,7S)-2,7-diacetoxynonane and 10 µg (2R,7R)-2,7-diacetoxynonane, or the solvent only control. A small number of female C. brassicola were caught (mean 0.9 females/trap/week; maximum 11 females on a single trap) and there were no significant differences in the numbers of females caught between treatments (Mixed Model, χ2 = 7.44, df = 5, P = 0.19).
Mean number (± 95% confidence interval) of male Contarinia brassicola caught per trap per week with dispensers loaded with five different ratios of two isomers of 2,7-diacetoxynonane and (2R)-2-acetoxynane plus an unbaited control in Experiment 2. Means are estimated marginal means based on the fixed effects of the mixed model used for analysis, with means and confidence intervals back-transformed from the logarithmic scale. Different letters indicate significant differences in numbers of midges caught (P < 0.05)
In Experiment 3, a significant overall difference was found between treatments in numbers of males caught (Mixed Model, χ2 = 308, df = 6, P < 0.001; Fig. 10), with differences between individual treatments detected through Tukey’s tests (P < 0.05). Traps baited with 10 µg (2R,7S) -2,7-diacetoxynonane plus 1.0 µg (2R,7R)-2,7-diacetoxynonane and 0.5 µg (2R)-2-acetoxynonane caught the most males and significantly more males than traps baited with 10 µg (2R,7S) -2,7-diacetoxynonane and 1.0 µg (2R,7R)-2,7-diacetoxynonane or 10 µg (2R,7S)-2,7-diacetoxynonane and 2.0 µg (2R,7R)-2,7-diacetoxynonane. Traps baited with the two latter treatments caught more males than traps baited with 10 µg (2R,7S)-2,7-diacetoxynonane and 0.5 µg (2R,7R) -2,7-diacetoxynonane, which in turn caught males than traps baited with 10 µg (2R,7S)-2,7-diacetoxynonane and 0.1 µg (2R,7R)-2,7-diacetoxynonane or 10 µg (2R,7S)-2,7-diacetoxynonane and 5 µg (2R,7R)-2,7-diacetoxynonane. There was no difference in the numbers of males caught in traps baited with lures loaded with 10 µg (2R,7S)-2,7-diacetoxynonane and 5 µg (2R,7R)-2,7-diacetoxynonane and the blank control. Few female C. brassicola were caught (mean 0.73 females/trap/week, maximum 10 females on a single trap), and there was no significant overall difference in the number of females caught between treatments (Mixed Model, χ2 = 4.8, df = 6, P = 0.57).
Mean number (± 95% confidence interval) of male Contarinia brassicola caught per trap per week with dispensers loaded with six different ratios of (2R,7S)- and (2R,7R)-2,7-diacetoxynonane and (2R)-2-acetoxynane and an unbaited control in Experiment 3. Means are estimated marginal means based on the fixed effects of the mixed model used for analysis, with means and confidence intervals back-transformed from the logarithmic scale. Different letters indicate significant differences in numbers of midges caught (P < 0.05)
Discussion
The results of this study demonstrated that virgin female C. brassicola produced a sex pheromone consisting of three components: (2R,7S)-2,7-diacetoxynonane, (2R,7R)-2,7-diacetoxynonane and (2R)-2-acetoxynonane. In field trap** tests, the individual components did not attract male C. brassicola, and the most attractive blend of those tested was similar to that produced by the female midges in a 100: 10: 5 ratio, respectively.
The major component of the pheromone of C. brassicola, 2,7-diacetoxynonane, has not been reported as a component of the sex pheromone of any other cecidomyiid midge, but has a structure consistent with those found in many other midge species (Hall et al. 2012; Xu et al. 2020; Mori et al. 2019). However, the availability of a pheromone trap will allow for future studies to determine if C. brassicola is found elsewhere throughout the world and may aid in the identification of other host plants. Studies have been commenced to determine the optimum trap design and placement for C. brassicola, and to attempt to correlate numbers of males captured in pheromone-baited traps with damage to canola in the field.
Data Availability
Raw chromatographic and trap** data are available on request from the authors.
Code Availability
R Code used for data analysis is available from authors on request.
References
Amarawardana L (2009) The chemical diversity of midge pheromones. PhD Thesis University of Greenwich, UK, p 184
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Boddum T, Skals N, Wiren M, Baur R, Rauscher S, Hillbur Y (2009) Optimisation of the pheromone blend of the swede midge, Contarinia nasturtii, for monitoring. Pest Manag Sci 65:851–856. https://doi.org/10.1002/ps.1762
Boddum T, Skals N, Hill SR, Hansson BS, Hillbur Y (2010) Gall midge olfaction: Pheromone sensitive olfactory neurons in Contarinia nasturtii and Mayetiola destructor. J Insect Physiol 56:1306–1314. https://doi.org/10.1016/j.**sphys.2010.04.007
Bruce TJA, Hooper AM, Ireland LA, Jones OT, Martin JL, Smart LE, Oakley J, Wadhams LJ (2007) Development of a pheromone trap monitoring system for orange wheat blossom midge, Sitodiplosis mosellana, in the UK. Pest Manag Sci 63:49–56
Bruschini C, Dani FR, Pieraccini G, Guarna F, Turillazzi S (2006) Volatiles from the venom of five species of paper wasps (Polistes dominulus, P. gallicus, P. nimphus, P. sulcifer and P. olivaceus). Toxicon 47:812–825
Campbell EO, Dupuis JR, Holowachuk J, Hladun S, Vankosky MA, Mori BA (2020) Disjunction between canola distribution and the genetic structure of its recently described pest, the canola flower midge (Contarinia brassicola). Ecol Evol 10:13284–13296. https://doi.org/10.1002/ece3.6927
Canola Council of Canada (2021) About Canola: Industry Overview https://www.canolacouncil.org/about-canola/industry/. Accessed 21 July 2021
Censier F, Fischer CY, Chavalle S, Heuskin S, Fauconnier M-L, Bodson B, De Proft M, Lognay GC, Laurent P (2014) Identification of 1-methyloctyl butanoate as the major sex pheromone component from females of the saddle gall midge, Haplodiplosis marginata (Diptera: Cecidomyiidae). Chemoecology 24:243–251. https://doi.org/10.1007/s00049-014-0167-0
Choi N, Khaskin G, Gries R, Gries G, Bernard DR, Raworth DA, Kim D, Bennett RG (2004) (2R,7S)-Diacetoxytridecane: sex pheromone of the aphidophagous gall midge, Aphidoletes aphidimyza. J Chem Ecol 30:659–670
Collins AM, Blum MS (1983) Alarm responses caused by newly identified compounds derived from the honeybee sting. J Chem Ecol 9:57–65
Gries R, Gries G, Khaskin G, King S, Olfert O, Kaminski L, Lamb R, Bennett R (2000) Sex pheromone of orange wheat blossom midge, Sitodiplosis mosellana. Naturwissenschaften 87:450–454. https://doi.org/10.1007/s001140050757
Gries R, Khaskin G, Gries G, Bennett RG, King GGS, Morewood P, Slessor KN, Morewood WDJ (2002) (Z, Z)-4,7-Tridecadien-(S)-2-yl acetate: sex pheromone of douglas-fir cone gall midge, Contarinia oregonensis. J Chem Ecol 28:2283–2297
Hall DR, Amarawardana L, Cross JV, Francke W, Boddum T, Hillbur Y (2012) The chemical ecology of cecidomyiid midges (Diptera: Cecidomyiidae). J Chem Ecol 38:2–22. https://doi.org/10.1007/s10886-011-0053-y
Hillbur Y, Anderson P, Arn H, Bengtsson M, Löfqvist J, Biddle AJ, Smitt O, Högberg HE, Plass E, Franke S, Francke W (1999) Identification of sex pheromone components of the pea midge, Contarinia pisi (Diptera: Cecidomyiidae). Naturwissenschaften 86:292–294
Hillbur Y, El-Sayed A, Bengtsson M, Lofqvist J, Biddle A, Plass E, Francke W (2000) Laboratory and field study of the attraction of male pea midges, Contarinia pisi, to synthetic sex pheromone components. J Chem Ecol 26:1941–1952. https://doi.org/10.1023/a:1005557026246
Hillbur Y, Bengtsson M, Lofqvist J, Biddle A, Pillon O, Plass E, Francke W, Hallberg E (2001) A chiral sex pheromone system in the pea midge, Contarinia pisi. J Chem Ecol 27:1391–1407. https://doi.org/10.1023/a:1010317310027
Hillbur Y, Celander M, Baur R, Rauscher S, Haftmann J, Franke S, Francke W (2005) Identification of the sex pheromone of the swede midge, Contarinia nasturtii. J Chem Ecol 31:1807–1828. https://doi.org/10.1007/s10886-005-5928-3
Hooper AM, Dufour S, Willaert S (2007) Synthesis of (2S,7S)-dibutyroxynonane, the sex pheromone of the orange wheat blossom midge, Sitodiplosis mosellana (Gehin) (Diptera : Cecidomyiidae), by diastereoselective silicon-tethered ring-closing metathesis. Tetrahedron Lett 34:5991–5994
Lenth R (2020) emmeans: Estimated marginal means, aka least-squares means, R package Version 1.1. https://CRAN.R-project.org/package=emmeans
Molnar BP, Boddum T, Hill SR, Hansson BS, Hillbur Y, Birgersson G (2018) Ecological and phylogenetic relationships shape the peripheral olfactory systems of highly specialized gall midges (Cecidomiiydae). Front Physiol 9:323. https://doi.org/10.3389/fphys.2018.00323
Mori BA, Andreassen L, Heal JD, Dupuis JR, Soroka JJ, Sinclair BJ (2019) A new species of Contarinia Rondani (Diptera: Cecidomyiidae) that induces flower galls on canola (Brassicaceae) in the Canadian prairies. Can Entomol 151:131–148. https://doi.org/10.4039/tce.2018.63
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-Plus. Springer, New York
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rowley C, Pope TW, Cherrill A, Leather SR, Fernández-Grandon GM, Hall DR (2017) Development and optimisation of a sex pheromone lure for monitoring populations of saddle gall midge, Haplodiplosis marginata (Diptera: Cecidomyiidae). Entomol Exp Appl 163:82–92. https://doi.org/10.1111/eea.12560
Rowley C, Cherrill AJ, Leather SR, Hall DR, Pope TW (2018) Factors affecting trap catch in pheromone-based monitoring of saddle gall midge Haplodiplosis marginata (Diptera: Cecidomyiidae). Pest Manag Sci 74:406–412. https://doi.org/10.1002/ps.4721
Stringham GR (1971) Genetics of four hypocotyl mutants in Brassica campestris L. J Hered 62:248–250
Vankosky MA, Hladun S, Soroka JJ, Andreassen L, Meers S, Mori BA (2022) Distribution and life history of Contarinia brassicola (Diptera: Cecidomyiidae) in canola (Brassica napus) grown on the Canadian Prairies. Agric For Entomol. https://doi.org/10.1111/afe.12509
Xu L, **e Y, Na RS, Li QX (2020) Mini-review: recent advances in the identification and application of sex pheromones of gall midges (Diptera: Cecidomyiidae). Pest Manag Sci 76:3905–3910. https://doi.org/10.1002/ps.5949
Acknowledgements
We are grateful for the assistance of Shane Hladun, Jennifer Holowachuk, Jonathon Williams, Stephanie Harris, Ross Weiss, Kosuke Saita, Justin Kim, Morgan Cunningham, Tia Montgrand, Sandra Younie, Nathan Heuver, and Aidan Hamilton during the course of this project, especially in assisting with fieldwork and sample processing. This project was funded by the Canola Agronomic Research Program of the Canola Council of Canada (CARP Grant 2017.12 to MAV and BAM) and their partners, the Alberta Canola Producers Commission (Alberta Canola) and the Saskatchewan Canola Development Commission (SaskCanola).
Funding
This project was funded by the Canola Agronomic Research Program of the Canola Council of Canada (CARP Grant 2017.12 to MAV and BAM) and their partners, the Alberta Canola Producers Commission (Alberta Canola) and the Saskatchewan Canola Development Commission (SaskCanola).
Author information
Authors and Affiliations
Contributions
BAM and MAV were the managers of the project and supervised the provision of insects and field trap** work. DPB managed work at NRI and carried out electrophysiological assays and analysis of field trap** data. DRH, SJH, and DIF carried out pheromone collection, analysis, synthesis, and preparation of lures at NRI. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Ethics Approval
Not required.
Consent to Participate
Not required.
Consent for Publication
All authors agreed to the submission of the final manuscript.
Conflicts of Interest/Competing Interests
None. Dr. Boyd Mori is not related to the late Prof Kenji Mori.
Additional information
This paper is dedicated to the memory of Professor Kenji Mori who taught us so much about the structure and stereochemistry of natural products and was a much-loved friend and colleague.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bray, D.P., Hall, D.R., Harte, S.J. et al. Components of the Female Sex Pheromone of the Newly-Described Canola Flower Midge, Contarinia brassicola. J Chem Ecol 48, 479–490 (2022). https://doi.org/10.1007/s10886-022-01369-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-022-01369-z