Abstract
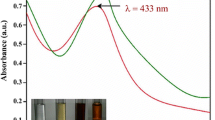
Stryphnodendron adstringens (Martius) Coville is a medicinal plant described as having pharmacological properties as anti-inflammatory and antimicrobial activities. Silver chloride nanoparticles (AgCl-NPs) have shown great potential for biomedical applications with efficient antimicrobial properties. Here, we report the photosynthesis of AgCl-NPs using plant extract from S. adstringens (SaAgCl-NPs) and their cytotoxic and antimicrobial activities. We photosynthesized SaAgCl-NPs nearly spherical with low heterogeneity in size and enveloped by an organic material layer responsible for colloidal stability. SaAgCl-NPs was non-cytotoxic against mammalian VERO cells; however, SaAgCl-NPs presented remarkable antifungal activity against the pathogenic yeast Cryptococcus neoformans (MIC80 of 0.32 µg/mL) the causative agent of human cryptococcosis. Notable antibacterial activity was observed against Gram-negative bacteria Pseudomonas aeruginosa (MIC80 of 2.56 µg/mL) e Serratia marcescens (MIC80 of 20.48 µg/mL) both microorganisms associated with a variety of human infections, in particular pneumonia. In contrast, Gram-positive bacteria Staphylococcus aureus and Staphylococcus epidermidis, microorganisms that cause pathologies as skin infections, were less susceptible to the SaAgCl-NPs both with MIC80 of 40.93 µg/mL. Thus, SaAgCl-NPs represents an organic–inorganic hybrid nanomaterial with very low cytotoxicity against mammalian cells and high antimicrobial efficiency against pathogenic microorganisms and may be explored as an alternative to antimicrobial drugs.





Similar content being viewed by others
References
E. Forero (1972). Brittonia 24 (2), 143–147. https://doi.org/10.2307/2805864.
E. A. Audi, D. P. Toledo, P. G. Peres, E. Kimura, W. K. V. Pereira, J. C. P. de Mello, C. Nakamura, W. Alves-do-Prado, R. K. N. Cuman, and C. A. Bersani-Amado (1999). Phytother. Res. 13 (3), 264–266.
G. C. Lopes, A. C. C. Sanches, C. V. Nakamura, B. P. Dias Filho, L. Hernandes, and J. C. P. Mello (2005). J. Ethnopharmacol. 99 (2), 265–272.
A. M. M. Felipe, V. P. Rincão, F. J. Benati, R. E. C. Linhares, K. J. Galina, C. E. de Toledo, G. C. Lopes, J. C. P. Mello, and C. Nozawa (2006). Biol. Pharm. Bull. 29 (6), 1092–1095.
S. C. G. Pinto, F. G. Bueno, G. P. Panizzon, G. Morais, P. V. P. dos Santos, M. L. Baesso, E. V. S. L. de Mello, and J. C. P. de Mello (2015). Planta Med. 81 (1), 1090–1096.
P. D. E. Mello, F. Petereit, and A. Nahrstedt (1996). Phytochemistry 41 (3), 807–813.
L. M. Ricardo and M. G. L. Brandão (2018). Springer Nat. 5, 431–437.
J. C. S. Lima, D. T. O. Martins, and P. T. de Souza (1998). Phytother. Res. 12, 218–220.
N. Durán, G. Nakazato, and A. B. Seabra (2016). Appl. Microbiol. Biotechnol. 100 (15), 6555–6570.
E. Lima, R. Guerra, V. Lara, and A. Guzmán (2013). Chem. Cent. J. 7 (1), 7:11.
A. Tofanello, J. N. Araujo, V. M. Silva, J. A. P. Sato, F. M. Squina, I. L. Nantes, and W. Garcia (2017). Int. J. Biol. Macromol. 102 (1), 84–91.
A. Tofanello, G. F. Cruz, J. N. Araújo, I. L. N. Cardoso, F. F. Ferreira, and W. Garcia (2018). J. Inorg. Org. Polym. Mater. 28 (5), 2056–2062.
J. N. Araújo, A. Tofanello, I. L. N. Cardoso, F. F. Ferreira, J. A. Souza, D. W. Lim, H. Kitagawa, and W. Garcia (2019). Colloids Surf. B 176, 47–54.
J. H. Kim, Y. C. Hong, and H. S. Uhm (2007). Surf. Coat. Technol. 201 (9–11), 5114–5120.
A. K. Gupta and M. Gupta (2005). Biomaterials 26 (18), 3995–4021.
S. H. Wu, C. Y. Mou, and H. P. Lin (2013). Chem. Soc. Rev. 42 (9), 3862–3875.
O. Gokcekaya, K. Ueda, K. Ogasawara, and T. Narushima (2018). Mater. Chem. Phys. J. 223 (1), 473–478.
R. Bigdelli, M. Shahnazari, E. Panahnejad, R. A. Cohan, A. Dashbolaghi, and V. Asgary (2019). Artif. Cells Nanomed. Biotechnol. 47 (1), 1603–1609.
D. Philip (2011). Spectrochim. Acta A 78 (1), 327–331.
V. K. Sharma, R. A. Yngard, and Y. Lin (2009). Adv. Colloid Interface Sci. 145 (1–2), 83–96.
S. Jayanthi, S. Shanthi, B. Vaseeharan, N. Gopi, M. Govindaranjan, N. S. Alharbi, S. Kadaikunnan, J. M. Khaled, and G. Benelli (2017). J. Photochem. Photobiol. B 170 (1), 208–216.
R. Sanghi and P. Verma (2009). Bioresour. Technol. 100 (1), 501–504.
L. Hernandes, L. M. S. da Pereira, F. Palazzo, and J. C. P. de Mello (1996). Br. J. Pharm. Sci. 46 (3), 30–40.
M. A. Madureira, C. M. de Junior, G. C. Freire, L. C. Carezzato, L. M. P. de Campos, and I. G. Pedron (2019). Int. J. Dent. Med. 4 (3), 45–51.
L. Ehret-Sabatier, D. Loew, M. Goyffon, P. Fehlbaum, J. A. Hoffmann, A. van Dorsselaer, and P. Bulet (1996). J. Biol. Chem. 271 (47), 29537–29544.
F. D. Silva, C. A. Rezende, D. C. Rossi, E. Esteves, F. H. Dyszy, S. Schreier, F. Gueiros-Filho, C. B. Campos, J. R. Pires, and S. Daffre (2009). J. Biol. Chem. 284 (50), 34735–34746.
E. Chaparro-Aguirre, P. J. Segura-Ramírez, F. L. Alves, K. A. Riske, A. Miranda, and P. I. Silva Júnior (2019). Sci. Rep. 9, 13631.
T. Rajan and S. Muthukrishnana (2013). Asian J. Pharm. Clin. Res. 6, 271–273.
M. M. R. Tejada, J. D. G. Durán, A. O. Ortega, M. E. Jimenez, R. P. Carpio, and E. Chibowski (2002). Colloid Surf. B 24 (3–4), 297–308.
R. M. Silverstein, F. X. Webste, D. J. Kimle, and D. L. Brice, Spectrometric Identification of Organic Compounds. (Wiley, Chichester, 2015), p. 466.
H. S. Mansur, C. M. Sadahira, A. N. Souza, and A. A. P. Mansur (2008). Mater. Sci. Eng. C 28 (4), 539–548.
P. Kumar, R. Yuvakkumar, S. Vijayakumar, and B. Vaseeharan (2018). Mater. Chem. Phys. 220 (1), 402–408.
S. Oliver, H. Wagh, Y. Liang, S. Yang, and C. Boyer (2018). J. Mater. Chem. B 6 (24), 4124–4138.
R. Bigdeli, M. Shahnazari, E. Panahnejad, R. A. Cohan, A. Dashbolaghi, and V. Asgary (2019). Artif. Cells Nanomed. Biotechnol. 47 (1), 1603–1609.
Y. Cheng, F. Wang, C. Fang, J. Su, and L. Yang (2016). J. Alloys Compd. 658 (1), 684–688.
T. Zhang, L. Wang, Q. Chen, and C. Chen (2014). Yonsei Univ. Coll. Med. J. 55 (2), 283–291.
V. T. Barbosa, J. K. C. Souza, V. Alvino, M. R. Meneghetti, P. P. F. Rodriguez, R. E. Moreira, G. V. B. Paulino, M. F. Landell, I. D. B. Júnior, T. G. do Nascimento, L. A. M. Grillo, and C. B. Dornelas (2019). Biotechnol. Prog. 35 (6), 1–9.
K. Ali, B. Ahmed, S. Dwivedi, Q. Saquib, A. A. Alkhedhairy, and J. Musarrat (2015). PLoS ONE 10 (7), 1–20.
A. Singh, P. K. Gautam, A. Verma, V. Singh, P. M. S. Priya, S. Shivalkar, and S. K. Samanta (2020). Biotechnol. Rep. 25 (1), 1–11.
A. Ahmad, Y. Wei, F. Syed, K. Tahir, A. U. Rehman, A. Khan, S. Ullah, and Q. Yuan (2017). Microb. Pathog. 102 (1), 133–142.
T. J. Silhavy, D. Kahne, and S. Walker (2010). Cold Spring Harb. Perspect. Biol. 2 (5), 1–16.
J. S. Kim, E. Kuk, K. N. Yu, et al. (2007). Nanomed. Nanotechnol. Biol. Med. 3 (1), 95–101.
M. Guzman, J. Dille, and S. Godet (2012). Nanomed. Nanotechnol. Biol. Med. 8 (1), 37–45.
R. Rajasingham, R. M. Smith, B. J. Park, J. N. Jarvis, N. P. Govender, T. M. Chiller, D. W. Denning, A. Loyse, and D. R. Boulware (2017). Lancet Infect. Dis. 17 (8), 873–881.
F. P. Gullo, S. A. Rossi, J. C. O. Sardi, V. L. I. Teodoro, M. J. S. M. Giannini, and A. M. F. Almeida (2013). Eur. J. Clin. Microbiol. Infect. Dis. 32 (11), 1377–1391.
European Centre for Disease Prevention and Control, ECDC (2015). https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf.
Acknowledgements
The authors are grateful to the Multiuser Central Facilities (UFABC).
Funding
We would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (Grants 2017/17275-3 and 2018/08194-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grants 305740/2017-2, 422132/2018-7, 404815/2018-9 and 313117/2019-5) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da S. Fernandes, D.G., Andrade, V.B., Lucena, L.N. et al. Cytotoxicity and Antimicrobial Properties of Photosynthesized Silver Chloride Nanoparticles Using Plant Extract from Stryphnodendron adstringens (Martius) Coville. J Clust Sci 33, 687–695 (2022). https://doi.org/10.1007/s10876-021-02011-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02011-w