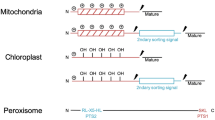
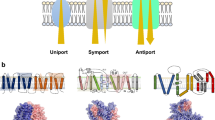
Today we know there are four different types of ATPases that operate within biological membranes with the purpose of moving many different types of ions or molecules across these membranes. Some of these ions or molecules are transported into cells, some out of cells, and some in or out of organelles within cells. These ATPases span the biological world from bacteria to eukaryotic cells and have become most simply and commonly known as “transport ATPases.” The price that each cell type pays for transport work is counted in molecules of hydrolyzed ATP, a metabolic currency that is itself regenerated by a transport ATPase working in reverse, i.e., the ATP synthase. Four major classes of transport ATPases, the P, V, F, and ABC types are now known. In addition to being involved in many different types of biological/physiological processes, mutations in these proteins also account for a large number of diseases. The purpose of this introductory article to a mini-review series on transport ATPases is to provide the reader with a very brief and focused look at this important area of research that has an interesting history and bears significance to cell physiology, biochemistry, immunology, nanotechnology, and medicine, including drug discovery. The latter involves potential applications to a whole host of diseases ranging from cancer to those that affect bones (osteoporosis), ears (hearing), eyes (macromolecular degeneration), the heart (hypercholesterolemia/cardiac arrest,), immune system (immune deficiency disease), kidney (nephrotoxicity), lungs (cystic fibrosis), pancreas (diabetes and cystic fibrosis), skin (Darier disease), and stomach (ulcers).
Similar content being viewed by others
References
Abrahams, J. B., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994). Nature (London) 370, 621–628.
Ames, G. F., and Roth, J. R. (1968). J. Bacteriol. 96, 1742–1749.
Amzel, L. M., McKinney, M., Narayanan, P., and Pedersen, P. L. (1982). Proc. Natl. Acad. Sci. U.S.A. 79, 5852–5856.
Amzel, L. M., and Pedersen, P. L. (1978). J. Biol. Chem. 253, 2067–2069.
Awayn, N. H., Rosenberg, M. F., Kamis, A. B., Aleksandrov, L. A., Riordan, J. R., and Ford, R. C. (2005). Biochem. Soc. Trans. 33, 996–999.
Bianchet, M., Amzel, L. M., Hullihen, J., and Pedersen, P. L. (1998). Proc. Natl. Acad. Sci. U.S.A. 95, 11065–11070.
Bianchet, M., Ysern, X., Hullihen, J., Pedersen, P. L., and Amzel, L. M. (1991). J. Biol. Chem. 266, 21197–21201.
Bowman, E. J., and Bowman, B. J. (1982). J. Bacteriol. 151, 1326–1337.
Brewer, H. B. Jr., Remaley, A. T., Neufeld, E. B., Basso, F., and Joyce, C. (2004). Arterioscler. Thomb. Vasc. Biol. 24, 1755– 1760.
Catterall, W. A., Coty, W. A., and Pedersen, P. L. (1973). J. Biol. Chem. 248, 7427–7431.
Chang, G., and Roth, C. B. (2001). Science 293, 1793–1800.
Charnock, J. S., Rosenthal, A. S., and Post, R. L. (1963). Aust. J. Exp. Biol. Med. 41, 675–686.
Chen, C., Ko, Y. H., Delannoy, M., Ludke, S. J., Chiu, W., and Pedersen, P. L. (2004). J. Biol. Chem. 279, 31761–31768.
Chen, J., Lu, G., Lin, J., Davidson, A. L., and Quiocho, F. A. (2003). Mol. Cell 12, 651–661.
Dean, M., and Annilo, T. (2005). Annu. Rev. Genomics Hum. Genet. 6, 123–142.
Forgac, M. (2000). J. Exp. Biol. 203, 71–80.
Gibbons, C., Montgomery, M. G., Leslie, A. G., and Walker, J. E. (2000). Nat. Struc. Biol. 7, 1055–1061.
Gottesman, M. M., and Ambudkar, S. (2001). J. Bioenerg. Biomembr. 33, 453–458.
Gresser, M. J., Meyers, J., and Boyer, P. D. (1982). J. Biol. Chem. 257, 12030–12038.
Higgins, C. F. (1992). Annu. Rev. Cell Biol. 8, 67–113.
Higgins, C. F., Hiles, I. D., Salmond, G. P., Gill, D. R., Downie, J. A., Evans, I. J., Holland, I. B., Gray, L., Buckel, S. D., Bell, A. W., and Hermodson, M. A. (1986). Nature 323, 448–450.
Hirata, T., Iwamoto-Kihara, A., Sun-Wada, G. H., Okajima, T., Wada, Y., and Futai, M. (2003). J. Biol. Chem. 278, 23714– 23719.
Hyde, S. C., Emsley, P., Hartshorn, M., Mimmack, M. M., Gileadi, U., Pearce, S. R., Gallagher, M. P., Gill, D. R., Hubbard, R. E., and Higgins, C. F., (1990). Nature (London) 346, 362– 365.
Imamura, H., Nakano, M., Noji, H., Muneyuki, E., Ohkuma, S., Yoshida, M., and Yokoyama, K. (2003). Proc. Natl. Acad. Sci. 100, 2312–2315.
Ko, Y. H., Delannoy, M., Hullihen, J., Chiu, W., and Pedersen, P. L. (2003). J. Biol. Chem. 278, 12305–12309.
Ko, Y. H., Smith, B. L., Wang, Y., Pomper, M. G., Rini, D. A., Torbenson, M. S., Hullihen, J., and Pedersen, P. L. (2004). Biochem. Biophys. Commun. 324, 269–275.
Langheim, S., Yu, L., von Bergmann, K., Lutjohann, D., Xu, F., Hobbs, H. H., and Cohen, J. (2005). J. Lipid Res. 46, 1732– 1738.
Lee, J. Y., and Parks, J. S. (2005). Curr. Opin. Lipidol. 16, 19– 25.
Locher, K. P. (2004). Curr. Opin. Struct. Biol. 14, 426–431.
Noji, H., Yasuda, R., Yoshida, M., and Kinosita, K., Jr. (1997). Nature 386, 299–302.
Ohsumi, Y., and Anraku, Y. (1981). J. Biol. Chem. 256, 2079–2082.
Pedersen, P. L., and Carafoli, E. (1987a). Trends in Biochem. Sci. 12, 146–150.
Pedersen, P. L., and Carafoli, E. (1987b). Trends in Biochem. Sci. 12, 186–189.
Pedersen, P. L., Ko, Y. H., and Hong, S. (2000). J. Bioenerg. Biomemb. 32, 423–432.
Pedersen, P. L. (2002). J. Bioenerg. Biomemb. 34, 327–332.
Post, R. L., and Jolly, P. C. (1957). Biochim. Biophys. Acta 25, 118– 128.
Post, R. L., Sen, A. K., and Rosenthal, A. S. (1965). J. Biol. Chem. 240, 1437–1445.
Pullman, M. E., Penefsky, H., and Racker, E. (1958). Arch. Biochem. Biophys. 76, 227–230.
Stock, D., Gibbons, C., Arechaga, I., Leslie, A. G., and Walker, J. E. (2000). Curr. Opin. Struct. Biol. 10, 672–679.
Stock, D., Leslie, A. G., and Walker, J. E. (1999). Science 286, 1700–1705.
Skou, J. C. (1957). Biochim. Biophys. Acta 23, 394–401.
Skou, J. C., and Esmann, M. (1992). J. Bioenerg. Biomemb. 24, 249– 261.
Toyoshima, C., and Inesi, G. (2004). Ann. Rev. Biochem. 73, 269–292.
Toyoshima, C., and Misutani, T. (2004). Nature 430, 529–535.
Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000). Nature 405, 647–655.
Weber, J., and Senior, A. E. (2003). FEBS Lett. 545, 61–70.
Wilkens, S., Zhang, Z., and Zheng, Y. (2005). Micron 36, 109– 126.
Yokoyama, K., Nakano, M., Imamura, H., Yoshida, M., and Tamakoshi, M. (2003). J. Biol. Chem. 278, 24255–24258.
Yoshida, M., Muneyuhi, E., and Hisabori, T. (2001). Nat. Rev. Mol. Cell Biol. 2, 669–677.
Yu, L., Gupta, S., Xu, F., Liverman, A. D., Moschetta, A., Mangelsdorf, D. J., Repa, J. J., Hobbs, H. H., and Cohen, J. C. (2004). J. Biol. Chem. 280, 8742–8747.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedersen, P.L. Transport ATPases: Structure, Motors, Mechanism and Medicine: A Brief Overview. J Bioenerg Biomembr 37, 349–357 (2005). https://doi.org/10.1007/s10863-005-9470-3
Issue Date:
DOI: https://doi.org/10.1007/s10863-005-9470-3