Abstract
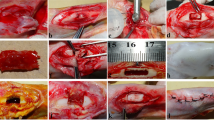
Osteomyelitis (bone infection) is often difficult to cure. The commonly-used treatment of surgical debridement to remove the infected bone combined with prolonged systemic and local antibiotic treatment has limitations. In the present study, an injectable borate bioactive glass cement was developed as a carrier for the antibiotic vancomycin, characterized in vitro, and evaluated for its capacity to cure osteomyelitis in a rabbit tibial model. The cement (initial setting time = 5.8 ± 0.6 min; compressive strength = 25.6 ± 0.3 MPa) released vancomycin over ~25 days in phosphate-buffered saline, during which time the borate glass converted to hydroxyapatite (HA). When implanted in rabbit tibial defects infected with methicillin-resistant Staphylococcus aureus (MRSA)-induced osteomyelitis, the vancomycin-loaded cement converted to HA and supported new bone formation in the defects within 8 weeks. Osteomyelitis was cured in 87 % of the defects implanted with the vancomycin-loaded borate glass cement, compared to 71 % for the defects implanted with vancomycin-loaded calcium sulfate cement. The injectable borate bioactive glass cement developed in this study is a promising treatment for curing osteomyelitis and for regenerating bone in the defects following cure of the infection.











Similar content being viewed by others
References
Ryf C, Goldhahn S, Radziejowski M, Blauth M, Hanson B. A new injectable brushite cement: first results in distal radius and proximal tibia fractures. Eur J Trauma Emerg S. 2009;35:389–96.
Schneider G, Blechschmidt K, Linde D, Litschko P, Körbs T, Beleites E. Bone regeneration with glass ceramic implants and calcium phosphate cements in a rabbit cranial defect model. J Mater Sci Mater Med. 2010;21:2853–9.
Liu X, Wang X, Chen Z, Cui F, Liu H, Mao K, Wang Y. Injectable bone cement based on mineralized collagen. J Biomed Mater Res B. 2010;94:77–9.
Cui G, Li J, Lei W, Bi L, Tang P, Liang Y, Tao S, Wang Y. The mechanical and biological properties of an injectable calcium phosphate cement-fibrin glue composite for bone regeneration. J Biomed Mater Res B. 2010;92:377–85.
Kuang G, Yau W, Lam W, Wu J, Chiu K, Lu W, Pan H. An effective approach by a chelate reaction in optimizing the setting process of strontium-incorporated calcium phosphate bone cement. J Biomed Mater Res B. 2012;100:778–87.
Ginebra MP, Fernandez E, De Maeyerl EAP, Verbeeckl RMH, Boltong MG, Ginebra J, Driessens FCM, Planell JA. Setting reaction and hardening of an apatitic calcium phosphate cement. J Dent Res. 1997;76:905–12.
Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 2000;31:SD37–47.
de Val Mate-Sanchez JE, Calvo-Guirado JL, Delgado-Ruiz RA, Ramirez-Fernandez MP, Martinez IM, Granero-Marin JM, Negri B, Chiva-Garcia F, Martinez-Gonzalez JM, de Aza PN. New block graft of α-TCP with silicon in critical size defects in rabbits: chemical characterization, histological, histomorphometric and micro-CT study. Ceram Int. 2012;38:1563–70.
Zhang X, Jia W, Gu Y, **ao W, Liu X, Wang D, Zhang C, Huang W, Rahaman MN, Day DE, Zhou N. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials. 2010;31:5865–74.
Domingues ZR, Cortés ME, Gomes TA, Diniz HF, Freitas CS, Gomes JB, Faria AMC, Sinisterra RD. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials. 2004;25:327–33.
Yao A, Wang D, Huang W, Fu Q, Rahaman MN, Day DE. In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J Am Ceram Soc. 2007;90:303–6.
Liu X, Huang W, Fu H, Yao A, Wang D, Pan H, Lu W, Jiang X, Zhang X. Bioactive borosilicate glass scaffolds: in vitro degradation and bioactivity behaviors. J Mater Sci Mater Med. 2009;20:1237–43.
Brown RF, Day DE, Day TE, Jung S, Rahaman MN, Fu Q. Growth and differentiation of osteoblastic cells on 13–93 bioactive glass fibers and scaffolds. Acta Biomater. 2008;4:387–96.
Jia W, Zhang X, Luo S, Liu X, Huang W, Rahaman MN, Day DE, Zhang C, **e Z, Wang J. Novel borate glass chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater. 2010;6:812–9.
Fu Q, Rahaman MN, Bal BS, Bonewald LF, Kuroki K, Brown RF. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J Biomed Mater Res A. 2011;95:164–71.
Fu Q, Rahaman MN, Bal BS, Bonewald LF, Kuroki K, Brown RF. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. II. In vitro and in vivo biological evaluation. J Biomed Mater Res A. 2011;95:172–9.
Huang W, Rahaman MN, Day DE, Li Y. Mechanisms for converting bioactive silicate, borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. Phys Chem Glasses B. 2006;47:647–58.
Huang W, Day DE, Kittiratanapiboon K, Rahaman MN. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J Mater Sci Mater Med. 2006;17:583–96.
Theiss F, Apelt D, Brand B, Kutter A, Zlinszky K, Bohner M, Matter S, Frei C, Auer JA, Rechenberg B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials. 2005;26:4383–94.
Moseke C, Saratsis V, Gbureck U. Injectability and mechanical properties of magnesium phosphate cements. J Mater Sci Mater Med. 2011;22:2591–8.
Bohner M. pH variations of a solution after injecting brushite cements. Key Eng Mater. 2000;192–195:813–6.
Lopez-Heredia MA, Kamphuis GJ, Thüne PC, Öner FC, Jansen JA, Walboomers XF. An injectable calcium phosphate cement for the local delivery of paclitaxel to bone. Biomaterials. 2011;32:5411–6.
**e Z, Liu X, Jia W, Zhang C, Huang W, Wang J. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate. J Control Release. 2009;139:118–26.
Jain AK, Panchagnula R. Skeletal drug delivery systems. Int J Pharm. 2000;206:1–12.
Cevhera E, Orhanb Z, Mülazımoğlu L, Şensoy D, Alper M, Yıldız A, Ozsoy Y. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int J Pharm. 2006;317:127–35.
Xu H, Weir MD, Burguera EF, Fraser AM. Injectable and macroporous calcium phosphate cement scaffold. Biomaterials. 2006;27:4279–87.
Kai D, Li D, Zhu X, Zhang L, Fan H, Zhang X. Addition of sodium hyaluronate and the effect on performance of the injectable calcium phosphate cement. J Mater Sci Mater Med. 2009;20:1595–602.
Norden CW, Myerowitz RL, Keleti E. Experimental osteomyelitis due to Staphylococcus aureus or Pseudomonas aeruginosa: a radiographic-pathological correlative analysis. Brit J Exp Pathol. 1980;61:451–60.
Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–4.
Khairoun I, Boltong MG, Driessens FM, Planell JA. Some factors controlling the injectability of calcium phosphate bone cements. J Mater Sci Mater Med. 1998;9:425–8.
Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(Suppl 2):S96–101.
Xu HHK, Weir MD, Simon CG. Injectable and strong nano-apatite scaffolds for cell-growth factor delivery and bone regeneration. Dent Mater. 2008;24:1212–22.
Goldstein SA. The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech. 1987;20:1055–61.
Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–75.
Zhao L, Weir MD, Xu HHK. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502–10.
Alkhraisat MH, Rueda C, Cabrejos-Azama J, Lucas-Aparicio J, Mariño FT, García-Denche JT, Jerez LB, Gbureck U, Cabarcos EL. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010;6:1522–8.
Ratier A, Gibson IR, Best SM, Freche M, Lacout JL, Rodriguez F. Setting characteristics and mechanical behaviour of a calcium phosphate bone cement containing tetracycline. Biomaterials. 2001;22:897–901.
Huang Y, Liu C, Shao H, Liu Z. Influence of tobramycin on properties of calcium phosphate cement. Chin J Biomed Eng. 2002;21:417–22.
Takechi M, Miyamoto Y, Ishikawa K, Nagayama M, Kon M, Asaoka K, Suzuki K. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. J Biomed Mater Res. 1998;39:308–16.
Muzzarelli RAA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci. 1997;53:131–40.
Zhang Y, Zhang M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J Biomed Mater Res. 2002;62:378–86.
Majeti NV, Ravi K. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27.
Jiang PJ, Patel S, Gbureck U, Caley R, Grove LM. Comparing the efficacy of three bioceramic matrices for the release of vancomycin hydrochloride. J Biomed Mater Res B. 2010;93:51–8.
Kimakhe S, Bohic S, Larrose C, Reynaud A, Pilet P, Giumelli B, Heymann D, Daculsi G. Biological activities of sustained polymyxin B release from calcium phosphate biomaterial prepared by dynamic compaction: an in vitro study. J Biomed Mater Res. 1999;47:18–27.
Reynolds PE. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol. 1989;8:943–50.
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100:5–28.
Zhang Z, Egaña JT, Reckhenrich AK, Schenck TL, Lohmeyer JA, Schantz JT, Machens HG, Schilling AF, et al. Cell-based resorption assays for bone graft substitutes. Acta Biomater. 2008;8:13–9.
Hench LL. Genetic design of bioactive glass. J Eur Ceram Soc. 2009;29:1257–65.
McAuliffe JA. Bone graft substitutes. J Hand Ther. 2003;16:180–7.
Fu H, Fu Q, Zhou N, Huang W, Rahaman MN, Wang D, Liu X. In vitro evaluation of borate-based bioactiveglass scaffolds prepared by a polymer foam replication method. Mater Sci Eng C. 2009;29:2275–81.
Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor ii mrna expression and protein synthesis. Biochem Biophys Res Com. 2000;24:461–5.
Jell G, Stevens MJ. Gene activation by bioactive glasses. J Mater Sci Mater Med. 2006;17:997–1002.
Gorustovich AA, Perio DDS, Boccaccini AR. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng B. 2010;16:199–207.
Acknowledgments
This work was supported by the National Natural Science Foundation of China through the Project 51072133, 51372170, 81000788, and 81201377, and by the Shanghai Science Committee through the Project 12JC1408500.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cui, X., Zhao, C., Gu, Y. et al. A novel injectable borate bioactive glass cement for local delivery of vancomycin to cure osteomyelitis and regenerate bone. J Mater Sci: Mater Med 25, 733–745 (2014). https://doi.org/10.1007/s10856-013-5122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-5122-z