Abstract
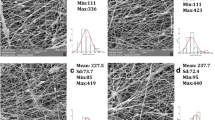
This study quantified the antibiotic release kinetics and subsequent bactericidal efficacy of rifampicin (RIF) against Gram-positive and Gram-negative bacteria under in vitro static conditions. Antibiotic-loaded scaffolds were fabricated by electrospinning poly(caprolactone) (PCL) with 10% or 20% (w/w) RIF. Scaffold fiber diameter and RIF loading were characterized, and RIF release kinetics were measured. RIF-releasing and RIF-free scaffolds were inoculated with Pseudomonas aeruginosa and Staphylococcus epidermidis, and the suspended concentration live and dead bacteria were determined by fluorescent microscopy. Adherent bacteria and biofilm formation were examined using scanning electron microscopy. Mean fiber diameters were 557 ± 399 nm for RIF-free, 402 ± 225 nm for 10% RIF, and 665 ± 402 nm for 20% RIF scaffolds. RIF release kinetics exhibited a short-burst release during the first hour, followed by a 7 h, zero-order release during which both RIF scaffolds released ~50% of their initial RIF mass loading. P. aeruginosa and S. epidermidis suspended cell populations proliferated in accordance with logarithmic growth models when exposed to control scaffolds; however both RIF-containing scaffolds completely inhibited bacterial growth in suspension and, subsequently, prevented biofilm formation within the scaffolds through the first 6 h.






Similar content being viewed by others
References
Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984–91.
Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87(8):1746–51.
Mont M, Waldman B, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection: a comparison-group study. J Bone Joint Surg. 2000;82:1552–7.
Shi Z, Neoh KG, Kang ET, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials. 2006;27(11):2440–9.
Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–71.
Moyad TF, Thornhill T, Estok D. Evaluation and management of the infected total hip and knee. Orthopedics. 2008;31(6):581–8.
Lee J, Singletary R, Schmader K, Anderson DJ, Bolognesi M, Kaye KS. Surgical site infection in the elderly following orthopaedic surgery. J Bone Joint Surg. 2006;88:1705–12.
Lee JH, Wang H, Kaplan JB, Lee WY. Microfluidic approach to create 3D tissue models for biofilm-related infection of orthopaedic implants. Tissue Eng Part C Methods. 2010.
Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27(11):2331–9.
Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol. 2010;59:227–38.
Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–9.
Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–9.
Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res. 2009;91B:470–80.
Gillespie WJ, Walenkamp GH. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2010;3:CD000244.
Chu VH, Crosslin DR, Friedman JY, Reed SD, Cabell CH, Griffiths RI, et al. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med. 2005;118(12):1416.
Bliziotis IA, Ntziora F, Lawrence KR, Falagas ME. Rifampin as adjuvant treatment of Gram-positive bacterial infections: a systematic review of comparative clinical trials. Eur J Clin Microbiol Infect Dis. 2007;26(12):849–56. doi:10.1007/s10096-007-0378-1.
Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19(4):349–56.
van de Belt H, Neut D, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. Acta Orthop Scand. 2001;72:557–71.
Nablo BJ, Prichard HL, Butler RD, Klitzman B, Schoenfisch MH. Inhibition of implant-associated infections via nitric oxide release. Biomaterials. 2005;26(34):6984–90.
Ko EK, Jeong SI, Rim NG, Lee YM, Shin H, Lee BK. In vitro osteogenic differentiation of human mesenchymal stem cells and in vivo bone formation in composite nanofiber meshes. Tissue Eng Part A. 2008;14(12):2105–19.
Ruckh TT, Kumar K, Kipper MJ, Popat KC. Osteogenic differentiation of bone marrow stromal cells on poly(epsilon-caprolactone) nanofiber scaffolds. Acta Biomater. 2010;6(8):2949–59.
Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–82.
Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006.
Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials. 2008;29(5):587–96.
Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009;5(7):2570–8. doi:10.1016/j.actbio.2008.12.013.
Hartman O, Zhang C, Adams EL, Farach-Carson MC, Petrelli NJ, Chase BD, et al. Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model. Biomaterials. 2010;31(21):5700–18.
Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98(1):47–56.
de Vries LE, Vallès Y, Agersø Y, Vaishampayan PA, García-Montaner A, Kuehl JV, et al. The gut as reservoir of antibiotic resistance: microbial diversity of tetracycline resistance in mother and infant. PLoS ONE. 2011;6(6):e21644.
Guarino V, Taddei P, Di Foggia M, Fagnano C, Ciapetti G, Ambrosio L. The influence of hydroxyapatite particles on in vitro degradation behaviour of Pcl based composite scaffolds. Tissue Eng Part A. 2009;15:3655–68.
Ritger P, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, and cylinders or discs. J Control Release. 1987;5:23–36.
Sinclair G, Peppas N. Analysis of non-Fickian transport in polymers using simplified exponential expressions. J Membr Sci. 1984;17:329–31.
Lavicky J, Urbanova Z, Raskova H, Rotta J, Vanecek J. The effects of peptidoglycan, a pyrogenic constituent of gram-positive microorganisms, on the pharmacokinetics of rifampicin. Toxicon. 1988;26(3):293–300.
Pohlod DJ, Saravolatz LD, Somerville MM. In vitro susceptibility of gram-positive cocci to LY146032 teicoplanin, sodium fusidate, vancomycin, and rifampicin. J Antimicrob Chemother. 1987;20(2):197–202.
Hoshino A, Isono Y. Degradation of aliphatic polyester films by commercially available lipases with special reference to rapid and complete degradation of poly(l-lactide) film by lipase PL derived from Alcaligenes sp. Biodegradation. 2002;13(2):141–7.
Zeng J, Chen X, Liang Q, Xu X, **g X. Enzymatic degradation of poly(l-lactide) and poly(epsilon-caprolactone) electrospun fibers. Macromol Biosci. 2004;4(12):1118–25.
Pitt C, Gratzl M, Kimmel C, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (dl-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2(4):215–20.
Pulkkinen M, Malin M, Tarvainen T, Saarimäki T, Seppälä J, Järvinen K. Effects of block length on the enzymatic degradation and erosion of oxazoline linked poly-ε-caprolactone. Eur J Pharm Sci. 2007;31(2):119–28.
Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17–28.
Bashur CA, Dahlgren LA, Goldstein AS. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d, l-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27(33):5681–8.
Venugopal J, Prabhakaran MP, Low S, Choon AT, Deepika G, Dev VR, et al. Continuous nanostructures for the controlled release of drugs. Curr Pharm Des. 2009;15(15):1799–808.
Bjerketorp J, Jacobsson K, Frykberg L. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett. 2004;234(2):309–14.
Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010;6(8):e1001036.
Liu L, Xu Y, Shea C, Fowler JS, Hooker JM, Tonge PJ. Radiosynthesis and bioimaging of the tuberculosis chemotherapeutics isoniazid, rifampicin and pyrazinamide in baboons. J Med Chem. 2010;53(7):2882–91.
Frippiat F, Meunier F, Derue G. Place of newer quinolones and rifampicin in the treatment of Gram-positive bone and joint infections. J Antimicrob Chemother. 2004;54(6):1158.
Pohlod DJ, Saravolatz LD, Somerville MM. In vitro susceptibility of Gram-positive cocci to LY146032 teicoplanin, sodium fusidate, vancomycin, and rifampicin. J Antimicrob Chemother. 1987;20:197–202.
Tupin A, Gualtieri M, Roquet-Baneres F, Morichaud Z, Brodolin K, Leonetti JP. Resistance to rifampicin: at the crossroads between ecological, genomic and medical concerns. Int J Antimicrob Agents. 2010;35(6):519–23.
Jatsenko T, Tover A, Tegova R, Kivisaar M. Molecular characterization of Rifr mutations in Pseudomonas aeruginosa and Pseudomonas putida. Mutation Res. 2010;683:106–14.
Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–54.
**ng Z-C, Chae W-P, Baek J-Y, Choi M-J, Jung Y, Kang I-K. In vitro assessment of antibacterial activity and cytocompatibility of silver-containing PHBV nanofibrous scaffolds for tissue engineering. Biomacromolecules. 2010;11:1248–53.
Liu X, Lin T, Fang J, Yao G, Zhao H, Dodson M, et al. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. J Biomed Mater Res. 2010;94A:499–508.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. T. Ruckh, R. A. Floreani contributed equally to this publication.
Rights and permissions
About this article
Cite this article
Ruckh, T.T., Floreani, R.A., Carroll, D.A. et al. Antimicrobial effects of nanofiber poly(caprolactone) tissue scaffolds releasing rifampicin. J Mater Sci: Mater Med 23, 1411–1420 (2012). https://doi.org/10.1007/s10856-012-4609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4609-3