Abstract
We studied the conductive properties of ink coatings composed of a mixture of Ni–Ag core–shell nanoparticles (NPs) at the size 70 nm and 250 nm. The metallic ink films were deposited on glass substrates by using bar coating and screen printing methods. The effect of the type of deposition method of ink coatings, as well as the temperature and time of the sintering process on their conductivity, was investigated. The most conductive films were obtained after thermal sintering at 300 °C. The obtained conductivity was about 20% of that for a bulk nickel, more than 80% higher than for films formed with any single type of particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ongoing process of miniaturization and complexity of electronic devices requires searching for proper conductive materials. Therefore, in the last years, the methods of their preparation and application in the electronics industry have been extensively studied. In this context, conductive inks based on metallic nanoparticles (NPs) have attracted much attention. Currently, one of the most promising method of the fabrication of electronic circuits and devices is a printed technology, in which conductive inks containing metallic nanoparticles (NPs) are deposited on solid and/or flexible substrates for production of solar cells, thin film transistors, printed circuit boards, transparent conductive electrodes, flexible displays, electrochromic devices, or touch screens [1,2,3].
To formulate the conductive inks, several approaches have been used so far. They can be produced from organometallic compounds or conductive polymers, colloidal suspensions of metallic nanoparticles, or some combination of these components [4]. The conductive inks based on metallic NPs, due to their low melting point and high conductivity, are the most promising for the preparation of printed electronic circuits and devices. Particularly, silver or gold nanoparticles as high-performance electrical conductive materials, have been most commonly applied so far in the preparation of ink formulations [5,6,7]. Although silver and gold NPs have apparent advantages such as low resistivity and stability against oxidation process, they are expensive to use commercially. Besides, while using silver, the electro-migration process could lower the reliability of an electrical circuit. The high price of Ag NPs and the increasing requirements of reducing the production cost of electronic devices have led to searching for alternative nano-pigment for conductive ink preparation. Therefore, nickel nanoparticles [8, 9] are considered as a suitable replacement of gold and silver nanoparticles due to high conductivity, low price and diminished electro-migration process. The challenge with Ni NPs is their rapid oxidation in the atmospheric environment. Oxides are not conductive which delimit Ni NPs application in printed electronics fabrication. Therefore, the synthesis of Ni NPs, as well as conductive ink fabrication, requires the protection of those nanoparticles against the oxidation process. Coating the surface of Ni NPs by a silver layer, which results in the formation of nickel-silver core–shell structure, is an effective, fast and simple method to retard the oxidization process of the nickel core [10, 11].
Conventional methods of the fabrication of conductive tracks or electronic devices such as photolithography, vacuum deposition, and electroless plating processes have many disadvantages. They are multi-stage, expensive, and produce large amounts of waste. Therefore, in the last years, the alternative methods of manufacturing of conductive materials have been searched.
One of the fast and low-cost methods of deposition is bar coating, which provides a simple but effective application of paints, printing inks, lacquers and other surface coatings onto many substrates, including paper, plastic films, foils, metal plates, glass plates, etc. In a single operation, two or more layers can be applied side-by-side, which makes the technique ideal for comparing products [12, 13]. Another alternative deposition method is the screen printing of pastes composed of metallic micro- or nanoparticles. This technique is also fast and straightforward. It involves only two steps: printing and curing of the deposited pattern [13,14,15]. The main disadvantage of screen printing is the large amount of required ink, which generates high costs.
The process of formation of well-connected metallic nanoparticle networks in ink coatings is usually prevented by the presence of stabilizers and other ink composites (wetting agents, binders, defoamers, etc.), which create isolating layer between nanoparticles. Therefore, after the ink coating, the removal of the protective layer by post-coating treatments is usually required. To transform nonconductive ink coating into a conductive one, metallic nanoparticles need sintering to form a continuous network with direct contact between them. The unique properties of nanoparticles, such as high surface-to-volume ratio and enhanced self-diffusion of surface atoms, decrease their melting point [16, 17]. Therefore, the sintering temperature can be much lower than in the case of bulk material [18, 19]. The sintering process involves three stages and depends on the temperature and time of heating. The first stage significantly depends on the conditions of the process (the shorter time requires the higher temperature). In the second stage, which is important to obtain a high conductivity, the removal of the insulating protective particle layers and a dynamic change of the sheet resistance can be observed. In the last stage of sintering, the value of resistance is only slightly changed in a long time (a few hours) of heating. The sintering conditions play a significant role because they determine the conductivity of the coated films and the applicability of particular substrates. Therefore, they are important from the application point of view.
Besides of sintering conditions, nanoparticles properties such as particle size and shape influence on the final conductivity of deposited ink layer [16, 17]. Among them, particle size is one of the most important for ink coating to obtain high conductivity. The nano-size effect decreases the melting temperature of the metallic NPs in the ink coating. According to theoretical and experimental studies presented by Buffat et. al [20], the melting point of gold NPs significantly decreases when their size is smaller than 5–7 nm. Meng and Huang have observed the increase of resistivity that can be obtained under the same sintering conditions with increasing the diameter of the silver nanoparticles [21]. Besides, the conductivity of nanocomposite materials can be improved by using a mixture of nanoparticles of different sizes. It was found that for the ink coating based on the mixture of silver nanoparticles at the size 12 and 80 nm, the lowest value of resistivity has been obtained in the sintering temperature range 125–200 °C [22].
We recently reported [23] the preparation method of conductive inks based on Ni–Ag nanoparticles. The synthesized core–shell nanoparticles were stable against oxidation during the sintering process carried out at temperatures up to 350 °C. The conductivity of coating based on Ni–Ag NPs after sintering at 350 °C was 11% of that for a bulk nickel. Although the obtained conductivity is promising for the application of those ink in the fabrication of electronic tracks, in this work, we studied the dependence of conductive properties of ink coating based on the mixture of Ni–Ag NPs of two sizes 70 and 270 nm. We analyzed the effect of concentration of smaller and bigger core–shell NPs on printed film conductivity. We compared the value of resistivity of ink layers based on those nanoparticles obtained by using two different methods of coating (bar coating and screen printing). We found out that the mixing of Ni–Ag NPs at the size 70 and 250 nm (at mass ratio 1:1) resulted in a significant decrease in resistivity of coatings after sintering at 300 °C. The obtained conductivity was about 20% of that for a bulk nickel. This result with practical meaning can be explained by a larger number of contact points formed by nanoparticles with different size distribution, which leads to creating a more compact conductive network in the sintered ink coatings.
2 Materials and methods
2.1 Materials
Nickel (II) acetate tetrahydrate (Ni(CH3COO)2·4H2O), sodium borohydride (NaBH4), polyvinylpyrrolidone (PVP) MW 360000 polyacrylic acid (PAA) MW 100000 and glycerol were purchased from Sigma-Aldrich. The other used chemicals were silver nitrate (AgNO3) (CHEMPUR, Poland) and ammonia hydroxide (NH4OH) (Avantor, Poland). The wetting agent TEGO WET KL 245 (polyether siloxane copolymer) was supplied from Evonik (Germany) company. All solutions were prepared with the use of distilled water.
2.2 Synthesis of Ni–Ag nanoparticles
The Ni–Ag NPs with diameters of about 70 (Ni–Ag-70) and 250 nm (Ni–Ag-250) were synthesized by a two-step process described previously [24]. At the first stage, nickel ions, supplied in the form of nickel acetate (0.01M) or nickel–ammonia complex (0.1M), were reduced by the excess (0.015M) or deficiency (0.025M) of sodium borohydride, respectively. Polyacrylic acid (PAA) at the molecular weight 100000 kDa (1:1 molar ratio Ni:PAA) was used as the stabilizer. At the next step, AgNO3 or Ag–NH3+ (as the precursors of the silver shell) were added to the suspensions of Ni NPs (1:0.1 Ni:Ag molar ratio). After that, the transmetalation process took place, resulting in the formation of a core–shell structure. Further details of the preparation procedure are given in the previous paper [24] in the section “Materials and methods”.
2.3 Formulation of Ni–Ag-based inks
To prepare the ink based on the mixture of Ni–Ag NPs, first, their dispersions were concentrated by centrifugation process (15 min, 7000 rpm) to the final (the highest) concentration of nanoparticles about 15% w.w. The concentrated nanoparticles were stable against aggregation process as presented in our previous paper [24]. Then, the dispersions of smaller and bigger Ni–Ag NPs were mixed at different ratios. Then the properties of water-based Ni–Ag ink were optimized to prepare the films by using the bar coating as well as the screen printing method. The optimal properties (uniformity and homogeneity) of ink coatings for bar coating were obtained for a formulation containing TEGO WET KL 245 as a wetting agent at the concentration of 0.02%. To deposit Ni–Ag ink by a screen printing method, its viscosity was increased by using glycerol as a solvent and PVP 360000 at a 3% concentration.
2.4 Fabrication of metallic films and sintering process
Conductive metallic coatings were formed by the deposition of the inks on a glass substrate (3.5 × 2.5 cm). The glass was cleaned before ink deposition by washing with distilled water and isopropanol. The metallic films were fabricated by using two different methods: bar coating (with 0.05 mm of wire winding rod) [12] and screen printing [14]. After the coating, the ink layer was dried on a hot plate at 60ºC for 10 min. The sintering of metallic layers was performed on a hot plate at various temperatures (250–300ºC) and time (15–60 min) at the atmospheric environment.
2.5 Instrumentation
The particles hydrodynamic size distribution was determined by the dynamic light scattering (DLS) with cumulants analysis and the zeta potential value by the microelectrophoretic method using Zetasizer Nano Series from Malvern Instruments. Each value was obtained as an average of three subsequent runs of the instrument with at least 20 measurements. All experiments were performed in water at 25 °C. The morphology and topography of metallic coatings were studied by using Scanning Electron Microscopy (LEO Gemini 1530, Zeiss). The measurement of the thickness of the conductive layers was performed by energy-dispersive X-ray fluorescence (EDXRF, FISCHERSCOPE X-RAY XDL 230) method. The size of the analyzed area was 140 × 80 µm. The measurement was performed at least 10 times at different places of each sample. The chemical composition of formed coatings was analyzed by X-ray photoelectron spectroscopy (XPS) with an ESCA/XPS equipped with a semispherical analyzer EA15 (Prevac) using Al-Kα (1486.6 eV) radiation with a power of 180 W. The resolution of the spectrometer for the Ag 3d5/2 line was 0.9 eV, and the spectra were acquired at a pressure of 2 × 10− 9 mbar. The ink formulation was deposited by using a hand coater (Kontech, Poland) and a screen printer (Dyenamo, Sweden). The value of sheet resistance was measured with the four-point probe electrodes method (Milliohm Meter, Extech Instruments). The measurements were repeated five times for each sample. To calculate the resistivity, the value of sheet resistance was multiplied by the thickness [25] and the conductivity was the value reciprocal to the resistivity.
3 Results and discussion
Metallic ink for preparation of conductive tracks or electronic devices is composed of functional nanomaterial, aqueous or organic liquid vehicle, and various additives to provides optimal conditions of the coating process and good quality of conductive patterns [26].
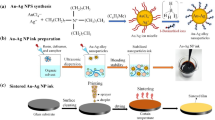
The conductive coatings based on Ni–Ag core–shell were prepared according to the scheme presented in Fig. 1. In the first step of their preparation, two types of Ni–Ag core–shell NPs at the hydrodynamic size 70 nm and 250 nm with the polydispersity index PDI < 0.21 were synthesized, as was presented in our previous paper [24]. The particle size for the inkjet technique should not be larger than 0.1 of the printhead’s orifice diameter (20–50 µm) to avoid it clogging and blockage [27]. Next, the synthesized NPs were concentrated by centrifugation process to obtain a formulation at a high concentration of NPs. In the third step, the dispersions of Ni–Ag-70 and Ni–Ag-250 NPs were mixed at different mass ratios (Ni–Ag-70:Ni–Ag-250: 1:0.5; 1:1; 0.5:1).
Figure 2 presents the size and zeta potential distribution of the dispersion of a mixture of Ni–Ag-70 and Ni–Ag-250 NPs at mixing ratio 1:1. Their values were similar for all studied ratios of those NPs. The size distribution (Fig. 2a, red and green curves for smaller and bigger NPs, respectively) shows the presence of bimodal nanoparticles. The determined zeta potential (as an average value of three subsequent runs of the instrument, Fig. 2b) of the dispersion of bimodal nanoparticles of − 39 ± 3 mV was close to values of NPs at the same size (− 45 ± 10 mV and − 30 ± 5 mV, respectively, for smaller and bigger NPs [24]) and indicated electrostatic stabilization of Ni–Ag NPs against the aggregation process, which is especially important for their application in the preparation of inkjet ink [27]. The increasing of the value of a polydispersity index (PDI) of the dispersion of Ni–Ag NPs from 0.21 ± 0.02 (similar for both sizes of Ni–Ag NPs) to 0.53 ± 0.05 after the mixing of particles at the different size indicated the formation of bimodal (more polydisperse) suspension of nanoparticles.
After the mixing of Ni-Ag-70 and Ni-Ag-250 NPs, the properties of ink formulation were optimized for the coating on a glass substrate by using a bar coating or screen printing method. The selection of the proper method of coating is essential for future conductive ink applications. In the last step of preparation of conductive tracks, deposited films of ink containing NPs were dried and finally, the thermal sintering was performed.
Viscosity and wettability [28] of the conductive ink formulation affect metallic coatings quality, which is crucial for obtaining high electrical conductivity. Therefore, in the next step of the fabrication of conductive coatings, the properties of metallic based ink formulation were optimized. It was presented in our previous work that for the case of individual suspensions the most uniform coating was obtained (by using hand coater) for TEGO WET KL 245 (as wetting agent) at concentration 0.01% and 0.025% for the ink based on 70 nm (Fig. 3a) and 250 nm (Fig. 3b) Ni–Ag NPs, respectively [24]. The optimal concentration of NPs for the ink containing the mixture of smaller and bigger core–shell NPs (for all mixing ratios) was 0.02%. Visually, the obtained layer was homogeneous; cracks or voids were not observed as it was shown in Fig. 3c. For the screen printing method, the viscosity of ink was increased by changing the solvent from water-based to glycerol-based. Moreover, PVP polymer was added at the selected concentration (3%) that increased viscosity as well as the wettability of ink formulation.
The morphology of ink coatings, composed of Ni–Ag-70 and Ni–Ag-250 NPs at mass ratio 1:0.5, 1:1 and 0.5:1, after the drying process (100 °C, 15 min) was studied by using SEM analysis. One can observe in Fig. 4, loosely packed nanoparticles at mixing ratio 1:0.5 and 0.5:1 of Ni–Ag-70 to Ni–Ag-250, whereas the coating with ink containing the Ni–Ag-70 and Ni–Ag-250 NPs at equal mass ratio (1:1), shows the more compact structure with single voids.
Although the metallic nanoparticles in the ink formulation have good conductivity, the coated films do not conduct an electric current due to the presence of organic stabilizers at their surface preventing metal–metal close contacts. Therefore, the removing of the insulating layer of stabilizer from the surface of NPs by sintering and forming stable joints between them is required. In this context, ink coatings composed of Ni–Ag-70 and Ni–Ag-250 NPs at different mixing ratios and total concentration of NPs 15% were heated at 300 °C for 30 min. As can be seen in Fig. 5, the value of resistivity strongly depends on the concentration ratio of Ni–Ag NPs at the size of 70 nm and 250 nm. We found out that the lowest value of resistivity (34 ± 2 µΩ cm) for the ink coating composed of core–shell nanoparticles at concentration 50% of Ni–Ag-70 and 50% of Ni–Ag-250 NPs. It can be explained by better packing of NPs in ink coatings in comparison to other mass ratios of NPs, as was shown in Fig. 3. Therefore, this optimal mixing ratio (1:1) of it was chosen for the next experiments.
We investigated the effect of sintering temperature and time on the value of sheet resistance for the metallic coatings composed of the NPs mixture Ni–Ag-70/Ni–Ag-250 at optimal ratio (1:1), deposited by the bar coating and screen printing. Figure 6 shows the relationship between the sheet resistance, the sintering temperature and time of the deposited Ni–Ag layers by using hand coater (A) and screen printer (B). The values of sheet resistance of ink coatings obtained by both deposition methods strongly depend on the temperature as well as on the time of sintering. It appears that over the whole ranges of temperature and sintering time, lower values of the sheet resistance were observed for coatings obtained using bar coating than deposited by a screen printing method. That can be attributed to the presence of additional organic materials (PVP and glycerol) in ink formulation, which was prepared for coatings deposited by a screen printing method. The boiling points of glycerol and PVP are about 290 °C and 220 °C, respectively, thus, to obtain the close contact between nanoparticles in ink coating the higher temperature of heating in comparison to water-based ink (deposited by bar coating) was required. The most visible decrease of the sheet resistance from 60 ± 10 to 2.5 ± 0.5 Ω/□ and 80 ± 10 to 5 ± 1 Ω/□ for ink coatings obtained by bar coating and screen printing method, respectively, at the whole temperature range (250–350 °C) after 15 min of sintering was noticed. From 30 min of heating, the values of sheet resistance were decreased only at a lower temperature (250–280 °C). At higher temperatures, from 300 °C, the values of sheet resistance were almost unchanged, regardless of the method of coatings deposition. At lower temperatures of heating (250–280 °C), the value of sheet resistance decreased up to one hour of sintering. The lowest value of sheet resistance (~ 0.18 Ω/□) at the temperature of sintering 300 °C was achieved after 30 min for ink deposited by bar coating. Sintering at the higher temperature (350 °C), was required to achieve at the same time a similar value of sheet resistance for ink layer coated by a screen printer. Therefore, for further experiments, the ink coatings sintered for 30 min at 300 °C and 350 °C deposited by bar coating and screen printing method, respectively, were selected.
The dependence of sheet resistance on the temperature and time of sintering of ink coatings based on the mixture of smaller and bigger Ni–Ag NPs deposited by using bar coating (a) and screen printing (b). The inset in a shows the comparison of the dependence of sheet resistance on time of sintering for both methods of ink coatings at optimal sintering temperature: 300 °C (black circles – bar coating, black triangles – screen printing) and 350 °C (gray circles—bar coating, gray triangles – screen printing) for deposition by bar coating and screen printing method, respectively
The conductivity of metallic coating is characterized by the value of resistivity, which is related to sheet resistance and the thickness of the coated layer. Therefore, the measurements of the thickness of the ink layer were performed by using the EDXRF method. In Fig. 7, the thickness of the ink layer (composed of the mixture of Ni–Ag NPs) deposited by bar coating (A) and screen printing (B) after sintering for 30 min at 300 °C and 350 °C, respectively, is presented. As can be seen, the thickness of coated films was similar, about 2 µm; however, the layer obtained by bar coating is more uniform.
To calculate the resistivity, the thickness of deposited ink layers was multiplied by the value of sheet resistance [25]. Since for the optimal conditions of sintering (for 30 min at 300 °C for bar coating and 350 °C for screen printing method), the lowest values of sheet resistance were 171 ± 10 mΩ/□ and 185 ± 20 mΩ/□ for ink coatings deposited by bar coating and screen printing method, respectively (as presented in Fig. 6). The calculated value of resistivity for metallic films deposited by bar coating was about 34 µΩ cm, which corresponds to 20 ± 1% of bulk nickel, while for coatings obtained by screen printing the conductivity was only slightly lower (about 18 ± 1% that of a bulk nickel).
The conductivity of a metallic coating deposited by screen printing was obtained at a higher temperature of sintering. As nowadays, printed electronic devices based on plastic substrates, sensitive to high temperatures, attracted much attention; therefore, low temperature of sintering is essential in the potential application of metallic ink for the production of conductive tracks. In this context, for further characterization, the conductive films coated by using bar coating were selected. In comparison to the results, which we obtained in our previous work [23], the conductivity of ink coating based on individual Ni–Ag NPs (at the size of about 70 nm or 270 nm) was much lower than for the ink films containing bimodal NPs presented in this paper. The highest conductivity previously obtained was 11% for the ink coating based on bigger core–shell NPs after sintering at 350 °C. The application of Ni–Ag NPs with bimodal sizes for the conductive coating preparation provides their efficient packing with a high number of contact points [29, 30]. Besides, pore-grain structure in metallic films is changed [31]. Smaller nanoparticles fill the voids between larger nanoparticles, which result in better contact between them and higher conductivity.
The morphology of the most promising metallic coatings (composed of 1:1 ratio of Ni–Ag-70 and Ni–Ag-250 NPs) deposited by the bar coating method, after the sintering process (30 min) at a various range of temperatures was analyzed by SEM (Fig. 8). After the heating at 250 °C, the Ni–Ag NPs nanoparticles still did not undergo a complete annealing process, which results in a high value of sheet resistance (~ 46 Ω/□). The formation of the dense network percolation paths due to the sintering at 300 °C can be observed, as can be seen in Fig. 8. The nanoparticles were completely welded and coalesced with each other and created a continuous layer, which provided high conductivity, as is showed in Fig. 3. The similar morphological properties of the metallic coating could be observed after the sintering at 350 °C; therefore, the conductivity was close to obtained after the sintering at 300 °C.
The XPS spectra illustrated in Fig. 9 of Ni–Ag NPs ink film, deposited by bar coating, after the sintering process at optimal conditions (300 °C, 30 min) show peaks characteristic of both metals. It can be noted that the atomic percentage (~ 45%) of metallic Ni is similar to the one for the Ni–Ag NPs dispersion (~ 51% for bigger and ~ 33% for smaller NPs) measured directly after the synthesis process [24]. That indicates thermal stability against oxidation of nickel NPs due to the presence of a silver shell. In the XPS spectrum of silver (Fig. 9B), 96% (atomic percentage) of metallic silver was detected. That result is similar to one obtained directly after the synthesis of the core–shell structure [24], which also suggests that the heating does not affect the chemical stability of silver shells against the oxidation process.
XPS spectra of ink coating (composed of bimodal mixture of core–shell Ni–Ag) obtained after sintering process at 300 °C for 30 min: (a) analysis of nickel (spectra bands: metallic Ni—black curve, NiO—green curve, satellite—blue curve) (b) analysis of silver (spectra bands: metallic Ag—yellow curve, AgO—black curve)
4 Conclusions
The conductive ink formulation was prepared by using the mixture of Ni–Ag NPs with bimodal size distribution as a functional component. The wetting properties of ink were adjusted by using TEGO WET KL 245 as the wetting agent at a concentration of 0.02% that allowed obtaining uniform and homogenous ink coatings on the glass substrates. We found that at the 1:1 ratio of core–shell NPs at the size 70 nm and 270 nm coating with the most compact structure were received. The thermal sintering of films allowed obtaining films with high electrical conductivity. In particular, after the heating at 300 °C for 30 min, their conductivity was found to be 20% of one for bulk nickel, which was c.a. 80% higher than the one achieved for coating containing unimodal Ni–Ag NPs at the same size. A better packing of NPs in ink coating and a larger number of contact points between them can explain the obtained result in the case of the bimodal size distribution. Therefore, a more extensive conductive network in the sintered metallic films is created. We believe that such ink coatings with high conductivity can have great potential applications in the printed electronics industry.
References
K. Fukuda, T. Sekine, D. Kumaki, S. Tokito, Profile control of inkjet printed silver electrodes and their application to organic transistors. ACS Appl. Mater. Interfaces 5(9), 3916–3920 (2013)
M. Layani, A. Kamyshny, S.Magdassi, Transparent conductors composed of nanomaterials. Nanoscale 6(11), 5581–5591 (2014)
H. Jung, S.-H. Cho, J. Joung, Y.S. Oh, Studies on inkjet printed conducting lines for electronic devices. J. Electron. Mater. 36, 1211 (2007)
S.M. Bidoki, D.M. Lewis, M. Clark, A. Vakorov, P.A. Miller, D. McGorman Ink-jet fabrication of electronic components. J. Micromech. Microeng. 17(5), 967–974 (2007)
W. Shen, X. Zhang, Q. Huang, Q. Xu, W. Song, Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 6(3), 1622–1628 (2014)
M. Layani, M. Grouchko, S. Shemesh, S. Magdassi, Conductive patterns on plastic substrates by sequential inkjet printing of silver nanoparticles and electrolyte sintering solutions. J. Mater. Chem. 22, 14349–14352 (2012)
B.T. Anto, S. Sivaramakrishnan, L.-L. Chua, P.K.H. Ho, Hydrophilic sparse ionic monolayer-protected metal nanoparticles: highly concentrated nano-Au and nano-Ag ‘“inks”’ that can be sintered to near-bulk conductivity at 150 oC. Adv. Func. Mater. 20(2), 296–303 (2010)
S.H. Park, H.S. Kim, Flash light sintering of nickel nanoparticles for printed electronics. Thin Solid Films 550, 575–581 (2014)
Y. Kamikoriyama, H. Sawamoto, M. Horiuchi, Nickel ink and conductor film formed of nickel ink, US Patent Appl. US 2009/0252924, Pub. Date Oct. 8, 2009
J.J. **g, J. **e, G.Y. Chen, W.H. Li, M.M. Zhang, Preparation of nickel silver core–shell nanoparticles by liquid-phase reduction for use in conductive paste. J. Exp. Nanosci. 10(17), 1347–1356 (2015)
M.M. Mohammadi, S.S. Gunturi, S. Shao, S. Konda, R.D. Buchne, M.T. Swihart, Flame-synthesized nickel-silver nanoparticle inks provide high conductivity without sintering. Chem. Eng. J. 372, 648–655 (2019)
S. Naghdi, K.Y. Rhee, D. Hui, S.J. Park, A review of conductive metal nanomaterials as conductive, transparent, and flexible coatings, thin films, and conductive fillers: different deposition methods and applications. Coatings 8, 278 (2018)
W.J. Hyun, S. Lim, B.Y. Ahn, J.A. Lewis, C.D. Frisbie, L.F. Francis, Screen printing of highly loaded silver inks on plastic substrates using silicon stencils. ACS Appl. Mater. Interfaces 7, 12619–12624 (2015)
A. Kamyshny, S. Magdassi, Conductive nanomaterials for printed electronics. Small 10, 3515–3535 (2014)
J. Perelaer, P.J. Smith, D. Mager, D. Soltman, S.K. Volkman, V. Subramanian, J.G. Korvink, U.S. Schubert, Printed electronics: the challenges involved in printing devices, interconnects, and contacts based on inorganic materials. J. Mater. Chem. 20, 8446–8453 (2010)
M. Grouchko, P. Roitman, X. Zhu, I. Popov, A. Kamyshny, H. Su, S. Magdassi, Merging of metal nanoparticles driven by selective wettability of silver nanostructures. Nat. Commun. 5, 1–5 (2014)
Ch Chen, K. Suganuma, Microstructure and mechanical properties of sintered Ag particles withflake and spherical shape from nano to micro size. Mater. Des. 162, 311–321 (2019)
P. Buffat, J.P. Borel, Size effect on the melting temperature of gold particles. Phys. Rev. A 13, 2287–2298 (1976)
F. Meng, J. Huang, Evolution mechanism of photonically sintered nano-silver conductive patterns. Nanomaterials 9, 258 (2019)
K. Balantrapu, M. McMurran, D.V. Goia, Inkjet printable silver dispersions: effect of bimodal particle-size distribution on film formation and electrical conductivity. J. Mater. Res. 25(5), 821–827 (2010)
A. Pajor-Świerzy, R. Socha, R. Pawłowski, P. Warszynski, K. Szczepanowicz, Application of metallic inks based on nickel-silver core-shell nanoparticles for fabrication of conductive films. Nanotechnology 30, 225301 (2019)
A. Pajor-Świerzy, D. Gaweł, E. Drzymała, R. Socha, M. Parlińska-Wojtan, K. Szczepanowicz, P. Warszyński, The optimization of methods of synthesis of nickel-silver core-shell nanoparticles for conductive materials. Nanotechnology 30, 1–8 (2019)
A. Kamyshny, J. Steinke, S. Magdassi, Metal-based inkjet inks for printed electronics. Open Appl. Phys. J. 4, 19–36 (2011)
A. Kamyshny, S. Magdassi, Inkjet printing, in Kirk-Othmer Encyclopedia of Chemical Technology. (Wiley, Weinheim, 2013), pp. 1–21
A. Kamyshny, S. Magdassi, Metallic Nanoinks for Inkjet Printing of Conductive 2D and 3D Structures, in Nanomaterials for 2D and 3D Printing. ed. by S. Magdassi, A. Kamyshny (Wiley, Weinheim, 2017), pp. 119–140
S. Magdassi, Ink requirements and formulations guidelines, in The Chemistry of Inkjet Inks. ed. by S. Magdassi (World Scientific, New Jersey-LondonSingapore, 2010), pp. 19–41
R. German, Prediction of sintered density for bimodal powder mixtures. Metall. Mater. Trans. A 23, 1455 (1992)
R.L. Coble, Effects of particle-size distribution in initial-stage sintering. J. Am. Ceram. Soc. 56, 461 (1973)
J.M. Ting, R.Y. Lin, Effect of particle-size distribution on sintering. J. Mater. Sci. 29, 1867 (1994)
Acknowledgments
The financial support was received by the „Development of methodology of synthesis and stabilization of metal nanoparticles for conductive materials” project, which was carried out within the Homing/2016-3/28 programme of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund. Additional support of the statutory subsidy of the Ministry of Science and High Education for Jerzy Haber Institute of Catalysis and Surface Chemistry is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pajor-Świerzy, A., Pawłowski, R., Warszyński, P. et al. The conductive properties of ink coating based on Ni–Ag core–shell nanoparticles with the bimodal size distribution. J Mater Sci: Mater Electron 31, 12991–12999 (2020). https://doi.org/10.1007/s10854-020-03852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03852-3