Abstract
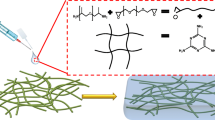
The ever-growing dendrite lithium is a hazardous behavior for lithium metal batteries due to triggering safety issues. Gel-based polymer electrolyte, which possesses high ionic conductivity, chemical stability, and high-level safety, has a great potential to substitute traditional liquid electrolyte to avoid electrolyte leakage, electrolyte decomposition, and interface issues. In this work, precursor solution which consists of polyethylene glycol 2000, isocyanate-ended tri-hydroxyl polyether polyols, and LiPF6 is in situ polymerized in SiO2 nanofiber membrane to prepare SiO2 nanofiber-supported organic–inorganic gel polymer electrolyte. In this polymer electrolyte, the inorganic SiO2 nanofiber framework is endued with good mechanical properties for polymer electrolyte, while the ether-rich polymer chains have superior compatibility with electrolyte, acting as ionic transportation network. The synergistic effect between organic and inorganic part induces uniform lithium deposition, thus resulting in remarkable lithium dendrite resistance and electrochemical performances including ionic conductivity, interfacial resistance, charge/discharge capacities, rate behavior, and long-term cyclic life span.





Similar content being viewed by others
Abbreviations
- PEG 2000:
-
Polyethylene glycol 2000
- THPP:
-
Tri-hydroxy polyether polyols
- SiO2-GPE:
-
SiO2 nanofiber-supported gel polymer electrolyte
- PVP:
-
Polyvinyl pyrrolidone
- TEOS:
-
Tetraethyl orthosilicate
- IPDI:
-
3-Isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate
- DBTL:
-
Dibutyltin dilaurate
- FT-IR:
-
Fourier-transform infrared spectroscopy
- TEM:
-
Transmission electron micrographs
- TGA:
-
Thermogravimetric analysis
- SEM:
-
Scanning electron microscopy
- EIS:
-
Electrochemical impedance spectroscopy
References
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Quartarone E, Mustarelli P (2011) Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem Soc Rev 40:2525–2540
Choi NS, Chen Z, Freunberger SA, Ji X, Sun YK, Amine K, Nazar LF, Cho J, Bruce PG (2012) Challenges facing lithium batteries and electrical double-layer capacitors. Angew Chem Int Ed Engl 51:9994–10024
Bai P, Li J, Brushett FR, Bazant MZ (2016) Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ Sci 9:3221–3229
Yang F, **e YY, Deng YL, Yuan C (2018) Predictive modeling of battery degradation and greenhouse gas emissions from US state-level electric vehicle operation. Nat. Commun. 9(2018):2429–2438
Zeng XX, Yin YX, Li NW, Du WC, Guo YG, Wan LJ (2016) Resha** lithium plating/strip** behavior via bifunctional polymer electrolyte for room-temperature solid Li metal batteries. J Am Chem Soc 138:15825–15828
Lin D, Liu Y, Cui Y (2017) Reviving the lithium metal anode for high-energy batteries. Nat Nanotechnol 12:194–206
**a M, Zhou Z, Su Y, Li Y, Wu Y, Zhou N, Zhang H, **ong X (2018) Scalable synthesis SiO@C anode by fluidization thermal chemical vapor deposition in fluidized bed reactor for high-energy lithium-ion battery. Appl Surf Sci 467:298–308
Liu K, Liu Y, Lin D, Pei A, Cui Y (2018) Materials for lithium-ion battery safety. Sci. Adv. 4:9820
Balakrishnan PG, Ramesh R, Prem Kumar T (2006) Safety mechanisms in lithium-ion batteries. J Power Sources 155:401–414
Goodenough JB, Kim Y (2009) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Jung J-W, Lee C-L, Yu S, Kim I-D (2016) Electrospun nanofibers as a platform for advanced secondary batteries: a comprehensive review. J. Mater. Chem. A 4:703–750
Khurana R, Schaefer JL, Archer LA, Coates GW (2014) Suppression of lithium dendrite growth using cross-linked polyethylene/poly (ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J Am Chem Soc 136:7395–7402
Wang Q, Yang S, Miao J, Zhang Y, Zhang D, Chen Y, Li Z (2018) Synthesis of graphene supported Li2SiO3/Li2SnO3 anode material for rechargeable lithium ion batteries. Appl Surf Sci 469:253–261
Chai J, Liu Z, Ma J, Wang J, Liu X, Liu H, Zhang J, Cui G, Chen L (2017) In-situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 4:1600377
Lu Q, Fang J, Yang J, Yan G, Liu S, Wang J (2013) A novel solid composite polymer electrolyte based on poly (ethylene oxide) segmented polysulfone copolymers for rechargeable lithium batteries. J Membr Sci 425:105–112
Huang Y, Zhong M, Huang Y, Zhu MS, Pei ZX, Wang ZF, Xue Q, **e XM, Zhi C (2015) A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte. Nat. Commun. 6:10310
Yu JL, Lu WB, Pei SP, Gong K, Wang LY, Meng LH, Huang YD, Smith JP, Booksh KS, Li QW, Byun JH, Oh Y, Yan YS, Chou TW (2016) Omnidirectionally stretchable high-performance supercapacitor based on isotropic buckled carbon nanotube films. ACS Nano 10:5204–5211
Nair JR, Destro M, Bella F, Appetecchi GB, Gerbaldi C (2016) Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J Power Sources 306:258–267
Lu Q, He Y-B, Yu Q, Li B, Kaneti YV, Yao Y, Kang F, Yang Q-H (2017) Dendrite-free, high-rate, long-life lithium metal batteries with a 3D cross-linked network polymer electrolyte. Adv Mater 29:1604460
Liu Ming, Zhou Dong, He Yan-Bing, Yongzhu Fu, Qin **anying, Miao Cui, Hongda Du, Li Baohua, Yang Quan-Hong, Zhiqun Lin TS, Zhao F Kang (2017) Novel gel polymer electrolyte for high-performance lithium-sulfur batteries. Nano Energy 22:278–289
Noto VD, Lavina S, Giffin GA, Negro E, Scrosati B (2011) Polymer electrolyte: present, past and future. Electrochim Acta 57:4–13
Dong T, Zhang J, Xu G, Chai J, Du H, Wang L, Zhou X (2018) A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energ Environ Sci 11:1197–1203
Duan H, Yin YX, Zeng XX, Li JY, Shi JL, Shi Y, Wan LJ (2018) In-situ plasticized polymer electrolyte with double-network for flexible solid-state lithium-metal batteries. Energy Storage Mater 10:85–91
Deng C, Jiang Y, Fan Z, Zhao S, Ouyang D, Tan J, Zhang P, Ding Y (2019) Sepiolite-based separator for advanced Li-ion batteries. Appl Surf Sci 484:446–452
Fan W, Li NW, Zhang X, Zhao S, Cao R, Yin Y, Li C (2018) A dual-salt gel polymer electrolyte with 3D cross-linked polymer network for dendrite-free lithium metal batteries. Adv Mater 5:1800559
Meyer WH (1998) Polymer electrolytes for lithium-ion batteries. Adv Mater 10:439–448
Liao H, Hong H, Zhang H, Li Z (2016) Preparation of hydrophilic polyethylene/methylcellulose blend microporous membranes for separator of lithium-ion batteries. J Membr Sci 498:147–157
Liao H, Zhang H, Hong H, Li Z, Qin G, Zhu H, Lin Y (2016) Novel cellulose aerogel coated on polypropylene separator as gel polymer electrolyte with high ionic conductivity for lithium-ion batteries. J Membr Sci 514:332–339
Liao H, Zhang H, Qin G, Li Z, Li L, Hong H (2017) A macro-porous graphene oxide-based membrane as a separator with enhanced thermal stability for high-safety lithium-ion batteries. RSC Adv 7:22112–22120
Zhai Y, **ao K, Yu J, Ding B (2015) Fabrication of hierarchical structure SiO2/Polyetherimide-polyurethane nanofibrous separators with high performance for lithium ion batteries. Electrochim Acta 154:219–226
Liang S, Shi Y, Ma T, Yan W, Qin S, Wang Y, Zhu Y, Wang H, Wu Y (2019) A compact gel membrane based on a blend of PEO and PVDF for dendrite-free lithium metal anodes. ChemElectroChem 6:1–8
Zhou D, Liu R, He Y-B, Li F, Liu M, Li B, Yang Q-H, Cai Q, Kang F (2016) SiO2 hollow nanosphere-based composite solid electrolyte for lithium metal batteries to suppress lithium dendrite growth and enhance cycle life. Adv Energy Mater 6:1502214
Zhou D, Tkacheva A, Tang X, Sun B, Shanmukaraj D, Li P, Zhang F, Armand M, Wang G (2019) Stable conversion chemistry-based lithium metal batteries enabled by hierarchical multifunctional polymer electrolytes with near-single ion conduction. Angew Chem Int Ed 58:6001–6006
Chen SM, Wang TL, Chang PY, Yang CH, Lee YC (2016) Poly(ionic liquid) prepared by photopolymerization of ionic liquid monomers as quasi-solid-state electrolytes for dye-sensitized solar cells. React Funct Polym 108:103–112
Diederichsen KM, Buss HG, Mccloskey BD (2017) The compensation effect in the Vogel–Tammann–Fulcher (VTF) equation for polymer-based electrolytes. Macromolecules 50:3831–3840
Hiller MM, Joost M, Gores HJ, Passerini S, Wiemhöfer HD (2013) The influence of interface polarization on the determination of lithium transference numbers of salt in polyethylene oxide electrolytes. Electrochim Acta 114:21–29
Liao H, Chen H, Zhou F, Zhang Z, Chen H (2019) Dendrite-free lithium deposition induced by mechanical strong sponge-supported unique 3D cross-linking polymer electrolyte for lithium metal batteries. J Power Sources 435:226748
Gao Y, Zhao Y, Li YC, Huang Q, Mallouk TE, Wang D (2017) Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J Am Chem Soc 139:15288–15291
Chen T, Kong W, Zhang Z, Wang L, Hu Y, Zhu G, Chen R, Ma L, Yan W, Wang Y, Liu J, ** Z (2018) Ionic liquid-immobilized polymer gel electrolyte with self-healing capability, high ionic conductivity and heat resistance for dendrite-free lithium metal batteries. Nano Energy 54:17–25
Wang H, Lin D, Liu Y, Li Y, Cui Y (2017) Ultrahigh-current density anodes with interconnected Li metal reservoir through overlithiation of mesoporous AlF3 framework. Sci. Adv. 3:e1701301
Acknowledgements
This work was supported by the link project of the National Natural Science Foundation of China (No. 11602082), Hunan Provincial Natural Science Foundation of China (Nos. 2017JJ3061 and 2019JJ50136) and the scientific research fundation of Hunan Provincial Education Department (Nos. 19C0596 and 19C596).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, H., Chen, H., Zhou, F. et al. A novel SiO2 nanofiber-supported organic–inorganic gel polymer electrolyte for dendrite-free lithium metal batteries. J Mater Sci 55, 9504–9515 (2020). https://doi.org/10.1007/s10853-020-04634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04634-2