Abstract
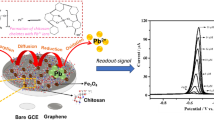
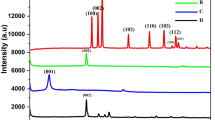
Herein, we report the one-pot solvothermal synthesis of mesoporous magnetite nanoparticle (m-Fe3O4)-loaded graphene oxide (GO) nanohybrid (m-Fe3O4/GO) and its utilization for the efficient electrochemical detection of hydrazine (HDZ). The as-synthesized m-Fe3O4/GO hybrid was characterized by transmission electron microscopy, powder X-ray powder diffraction, Fourier transform infrared spectroscopy, vibrating sample magnetometer, Brunauer–Emmett–Teller surface area and pore size analysis, X-ray photoelectron spectroscopy and thermogravimetric analysis. Electrochemical behaviour of the m-Fe3O4/GO was explored using electrochemical impedance spectroscopy, cyclic voltammetry and by amperometric responses. The results reveal that m-Fe3O4/GO hybrid-modified glassy carbon electrode (GCE) holds promising detection capability for HDZ with better features of the lower limit of detection (LOD), high sensitivity and extensive linear detection range compared to the bare GCE and GO-modified GCE. The values of LOD, sensitivity and linear detection range for m-Fe3O4/GO/GCE were 59 nM, 27 µA µM−1 cm−2 and 1–4400 µM, respectively. The high electron transfer rate and larger surface area of GO together with the mesoporous nature of Fe3O4 nanoparticles are responsible for the enhanced electrocatalytic activity of m-Fe3O4/GO-based electrochemical sensor. Most importantly, m-Fe3O4/GO/GCE-based electrochemical sensor developed in the present study exhibited excellent stability, reproducibility, reusability and anti-interference ability towards the detection of HDZ. The present study reveals that m-Fe3O4/GO is a promising material in develo** highly efficient electrochemical sensors and biosensors.













Similar content being viewed by others
References
Chabot V, Higgins D, Yu A, **ao X, Chen Z, Zhang J (2014) A review of graphene and graphene oxide sponge: material synthesis and applications to energy and the environment. Energy Environ Sci 7:1564–1596
Chen D, Tang L, Li J (2010) Graphene-based materials in electrochemistry. Chem Soc Rev 39:3157–3180
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Devi P, Sharma C, Kumar P, Kumar M, Bansod BKS, Nayak MK, Singla ML (2017) Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J Hazard Mater 322:85–94
Vinodha G, Cindrella L, Sithara V, Philip J, Shima PD (2018) Synthesis, characterization, thermal conductivity and rheological studies in magnetite-decorated graphene oxide nanofluids. J Nanofluids 7:1–10
Ganesan V, Louis C, Damodaran SP (2018) Novel nanofluids based on magnetite nanoclusters and investigation on their cluster size-dependent thermal conductivity. J Phys Chem C 122:6918–6929
Vellaichamy B, Periakaruppan P, Ponnaiah SK (2017) A new in situ synthesized ternary CuNPs-PANI-GO nano composite for selective detection of carcinogenic hydrazine. Sens Actuators B 245:156–165
Oh JA, Shin HS (2015) Simple and sensitive determination of hydrazine in drinking water by ultra-high-performance liquid chromatography–tandem mass spectrometry after derivatization with naphthalene-2,3-dialdehyde. J Chromatogr A 1395:73–78
Smolenkov AD, Rodin IA, Shpigun OA (2012) Spectrophotometric and fluorometric methods for the determination of hydrazine and its methylated analogues. J Anal Chem 67:98–113
Penneman RA, Audrieth LF (1948) Quantitative determination of hydrazine. Anal Chem 20:1058–1061
Safavi A, Karimi MA (2002) Flow injection chemiluminescence determination of hydrazine by oxidation with chlorinated isocyanurates. Talanta 58:785–792
Oha JA, Park JH, Shin HS (2013) Sensitive determination of hydrazine in water by gas chromatography–mass spectrometry after derivatization with ortho-phthalaldehyde. Anal Chim Acta 769:79–83
Karuppiah C, Palanisamy S, Chen S-M, Ramaraj SK, Periakaruppan P (2014) A novel and sensitive amperometric hydrazine sensor based on gold nanoparticles decorated graphite nanosheets modified screen printed carbon electrode. Electrochim Acta 139:157–164
Dong Y, Yang Z, Sheng Q, Zheng J (2018) Solvothermal synthesis of Ag@Fe3O4 nanosphere and its application as hydrazine sensor. Colloids Surf A 538:371–377
Wang C, Zhang L, Guo Z, Xu J, Wang H, Zhai K, Zhuo X (2010) A novel hydrazine electrochemical sensor based on the high specific surface area graphene. Microchim Acta 169:1–6
Wang L, Teng Q, Sun X, Chen Y, Wang Y, Wang H, Zhang Y (2018) Facile synthesis of metal-organic frameworks/ordered mesoporous carbon composites with enhanced electrocatalytic ability for hydrazine. J Colloid Interface Sci 512:127–133
Ambrosi A, Chua CK, Latiff NM, Loo AH, Wong CHA, Eng AYS, Bonanni A, Pumera M (2016) Graphene and its electrochemistry: an update. Chem Soc Rev 45:2458–2493
Deng K, Liu X, Li C, Hou Z, Huanga H (2017) A comparative study of different Fe3O4-functionalized carbon-based nanomaterials for the development of electrochemical sensors for bisphenol A. Anal Methods 9:5509–5517
Salamon J, Sathishkumar Y, Ramachandran K, Lee YS, Yoo DJ, Kim AR, GnanaKumar G (2015) One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens Bioelectron 64:269–276
Song H, Xue G, Zhang J, Wang G, Ye BC, Sun S, Tian L, Li Y (2017) Simultaneous voltammetric determination of dopamine and uric acid using carbon-encapsulated hollow Fe3O4 nanoparticles anchored to an electrode modified with nanosheets of reduced graphene oxide. Microchim Acta 184:843–853
Venosta L, Bracamontea MV, Rodriguezb MC, Jacoboc SE, Bercoffa PG (2017) Comparative studies of hybrid functional materials based on different carbon structures decorated with nano-magnetite. Suitable application as platforms for enzyme-free electrochemical sensing of hydrogen peroxide. Sens Actuators B 248:460–469
Sun Y, Zhang W, Yu H, Hou C, Li DS, Zhang Y, Liu Y (2015) Controlled synthesis various shapes Fe3O4 decorated reduced graphene oxide applied in the electrochemical detection. J Alloys Compd 638:182–187
Sun B, Gou X, Bai R, Abdelmoaty AAA, Ma Y, Zheng X, Hu F (2017) Direct electrochemistry and electrocatalysis of lobetyolin via magnetic functionalized reduced graphene oxide film fabricated electrochemical sensor. Mater Sci Eng C 74:515–524
Teymourian H, Salimi A, Khezrian S (2013) Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens Bioelectron 49:1–8
Arvand M, Hemmati S (2017) Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens Actuators B 238:346–356
Vennila P, Yoo DJ, Kim AR, Kumar GG (2017) Ni–Co/Fe3O4 flower-like nanocomposite for the highly sensitive and selective enzyme free glucose sensor applications. J Alloys Compd 703:633–642
Luo X, Pan J, Pan K, Yu Y, Zhong A, Wei S, Li J, Shi J, Li X (2015) An electrochemical sensor for hydrazine and nitrite based on graphene–cobalt hexacyanoferrate nanocomposite: toward environment and food detection. J Electroanal Chem 745:80–87
Zhao S, Wang L, Wang T, Han Q, Xu S (2016) A high-performance hydrazine electrochemical sensor based on gold nanoparticles/single-walled carbon nanohorns composite film. Appl Surf Sci 369:36–42
Shahid MM, Rameshkumar P, Basirunc WJ, Wijayantha U, Chiu WS, Khiew PS, Huang NM (2018) An electrochemical sensing platform of cobalt oxide@gold nanocubes interleaved reduced graphene oxide for the selective determination of hydrazine. Electrochim Acta 259:606–616
Dai G, **e J, Li C, Liu S (2017) Flower-like Co3O4/graphitic carbon nitride nanocomposite based electrochemical sensor and its highly sensitive electrocatalysis of hydrazine. J Alloys Compd 727:43–51
Meng Z, Liu B, Li M (2017) A sensitive hydrazine electrochemical sensor based on Ag–Ni alloy/reduced graphene oxide composite. Int J Electrochem 12:10269–10278
Deroco PB, Melo IG, Silva LSR, Eguiluz KIB, Banda GRS, Filho OF (2018) Carbon black supported Au–Pd core-shell nanoparticles within a dihexadecylphosphate film for the development of hydrazine electrochemical sensor. Sens Actuators B 256:535–542
Yaoyu Z, Lin T, **a X, Guangming Z, Jilai G, Danlian H, Yi Z, Guide Y, **g**g W, Chen Z (2015) A simple and sensitive hydrazine electrochemical sensor based on platinum nanoclusters. Curr Anal Chem 11:237–243
Zhao Z, Wang Y, Li P, Sang S, Zhang W, Hu J, Lian K (2015) A highly sensitive electrochemical sensor based on Cu/Cu2O@carbon nanocomposite structures for hydrazine detection. Anal Methods 7:9040–9046
Daemi S, Ashkarran AA, Bahari A, Ghasemi S (2017) Fabrication of a gold nanocage/graphene nanoscale platform for electrocatalytic detection of hydrazine. Sens Actuators B 245:55–65
He Y, Zheng J, Dong S (2012) Ultrasonic-electrodeposition of hierarchical flower-like cobalt on petalage-like graphene hybrid microstructures for hydrazine sensing. Analyst 137:4841–4848
Salimi A, Miranzadeh L, Hallaj R (2008) Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 75:147–156
Yang Z, Sheng Q, Zhang S, Zheng X, Zheng J (2017) One-pot synthesis of Fe3O4/polypyrrole/graphene oxide nanocomposites for electrochemical sensing of hydrazine. Microchim Acta 184:2219–2226
Zahrani EA, Soomro MT, Bashami RM, Rehman AU, Danish E, Ismail IMI, Aslam M, Hameed A (2016) Fabrication and performance of magnetite (Fe3O4) modified carbon paste electrode for the electrochemical detection of chlorite ions in aqueous medium. J Environ Chem Eng 4:4330–4341
Yao Y, Miao S, Liu S, Ma LP, Sun H, Wang S (2012) Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem Eng J 184:326–332
Shen X, Wu J, Bai S, Zhou H (2010) One-pot solvothermal syntheses and magnetic properties of graphene-based magnetic nanocomposites. J Alloys Compd 506:136–140
Wu ZS, Ren W, Wen L, Gao L, Zhao J, Chen Z, Zhou G, Li F, Cheng HM (2010) Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 4:3187–3194
Gong X-B (2016) Degradation of dye wastewater by persulfate activated with Fe3O4/graphene nanocomposite. J Water Reuse Desalination 06(4):553–561
Ojha DP, Joshi MK, Kim HJ (2017) Photo-Fenton degradation of organic pollutants using a zinc oxide decorated iron oxide/reduced graphene oxide nanocomposite. Ceram Int 43:1290–1297
Ganesan V, Louis C, Damodaran SP (2018) Graphene oxide-wrapped magnetite nanoclusters: a recyclable functional hybrid for fast and highly efficient removal of organic dyes from wastewater. J Environ Chem Eng 6:2176–2190
Lu J, Jiao X, Chen D, Li W (2009) Solvothermal synthesis and characterization of Fe3O4 and γ-Fe2O3 nanoplates. J Phys Chem C 113:4012–4017
Wang W, Tang B, Ju B, Gao Z, **u J, Zhang S (2017) Fe3O4-functionalized graphene nanosheet embedded phase change material composites: efficient magnetic and sunlight-driven energy conversion and storage. J Mater Chem A 5:958–968
Liu M, Chen C, Hu J, Wu X, Wang X (2011) Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J Phys Chem C 115:25234–25240
Wang D-W, Li F, Liu M, Lu GQ, Cheng HM (2008) 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew Chem 120:379–382
Yuan P, Fan M, Yang D, He H, Liu D, Yuan A, Zhu J, Chen T (2009) Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J Hazard Mater 166:821–829
Bhuvaneswari S, Pratheeksha PM, Anandan S, Rangappa D, Gopalan R, Rao TN (2014) Efficient reduced graphene oxide grafted porous Fe3O4 composite as a high performance anode material for Li-ion batteries. Phys Chem Chem Phys 16:5284–5294
Liu J, Shen J, Li M, Guo LP (2015) A high-efficient amperometric hydrazine sensor based on novel electrospun CoFe2O4 spinel nanofibers. Chin Chem Lett 26:1478–1484
Zhao J, Liu Z, Qin Y, Hu W (2014) Fabrication of Co3O4/graphene oxide composites using supercritical fluid and their catalytic application for the decomposition of ammonium perchlorate. CrystEngComm 16:2001–2008
Li X, Huang X, Liu D, Wang X, Song S, Zhou L, Zhang H (2011) Synthesis of 3D hierarchical Fe3O4/graphene composites with high lithium storage capacity and for controlled drug delivery. J Phys Chem C 115:21567–21573
** voltammetric and mutual interference analysis of Cd2+, Pb2+ and Hg2+ with reduced graphene oxide-Fe3O4 nanocomposites. Electrochim Acta 185:52–61
Rani GJ, Babu KJ, Kumar GG, Rajan MAJ (2016) Watsonia meriana flower like Fe3O4/reduced graphene oxide nanocomposite for the highly sensitive and selective electrochemical sensing of dopamine. J Alloys Compd 688:500–512
Yang YJ, Hu X, Xu Z (2017) CTAB assisted immobilization of RuO2 nanoparticles on graphene oxide for electrochemical sensing of hydrazine. Fuller Nanotubes Carbon Nanostruct 25:435–441
Ljukic BS, Banks CE, Crossley A, Compton RG (2006) Iron (III) oxide graphite composite electrodes: application to the electroanalytical detection of hydrazine and hydrogen peroxide. Electroanalysis 18:1757–1762
Li J, **e H, Chen L (2011) A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sens Actuators B 153:239–245
Li C, Li M, Bo X, Yang L, Mtukula AC, Guo L (2016) Facile synthesis of electrospinning Mn2O3-Fe2O3 loaded carbon fibers for electrocatalysis of hydrogen peroxide reduction and hydrazine oxidation. Electrochim Acta 211:255–264
Hu J, Zhang J, Zhao Z, Liu J, Shi J, Li G, Li P, Zhang W, Lian K, Zhuiykov S (2018) Synthesis and electrochemical properties of rGO-MoS2 heterostructures for highly sensitive nitrite detection. Ionics 24:577–587
Acknowledgements
Authors would like to thank the SAIF—CUSAT for XRD and TEM analysis, CIL—VISTAS for BET analysis and CARISM—Sastra University for XPS analysis. SPD also thanks Department of Science and Technology, Government of India for the INSPIRE Faculty Award and a research Grant (DST/INSPIRE/04/2014/001995).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vinodha, G., Shima, P.D. & Cindrella, L. Mesoporous magnetite nanoparticle-decorated graphene oxide nanosheets for efficient electrochemical detection of hydrazine. J Mater Sci 54, 4073–4088 (2019). https://doi.org/10.1007/s10853-018-3145-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-3145-z