Abstract
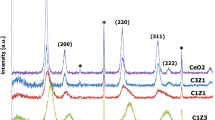
A series of ceria-zirconia (CZ) mixed oxides were prepared by coprecipitation, in which different amounts of H2O2 were added to investigate the influence of H2O2 on the properties of CZ. Modifications in structure, oxygen storage capacity, reducibility and thermal stability were studied systematically. The X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) results revealed that the thermal stability of the material was dramatically improved by the addition of H2O2, which was mainly due to the increase of the solubility of ZrO2 in ceria from 4.7 to 69.3%. The study of UV Raman spectroscopy confirmed that the concentration of defect sites of CZ materials increased with increasing H2O2/Ce molar ratio. The material prepared with H2O2/Ce molar ratio of 2 (CZ-2) exhibited the highest structural stability, the solubility of which was 68.8 and 69.3% before and after thermal treatment, and it maintained a homogeneous single cubic phase even after aging treatment at 1000 °C for 3 h. Also the highest thermal stability of the oxygen storage capacity (OSC) was found in CZ-2, which showed the reduction rates of 80.4 and 76.4% before and after thermal treatment. Moreover, a reaction mechanism of H2O2 and metal ions in the mixed precursor solution was assumed, in which the cerium-peroxide bridge-zirconium complex structure was proposed.










Similar content being viewed by others
References
Shinjo H (2006) Rare earth metals for automotive exhaust catalysts. J. Alloys Compd 37:1061–1064
Kašpar J, Fornasiero P, Graziani M (1999) Use of CeO2-based oxides in the three-way catalysis. Catal Today 50:285–298
Ozawa M, Kimura M, Isogai A (1993) The application of Ce-Zr oxide solid solution to oxygen storage promoters in automotive catalysts. J Alloys Compd 193:73–75
Mamontov E, Egami T, Brenzy R, Koranne M, Tyagi S (2000) Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J Phys Chem B 104:11110–11116
Wang Q, Li G, Zhao B, Zhou R (2011) The effect of rare earth modification on ceria–zirconia solid solution and its application in Pd-only three-way catalyst. J Mol Catal A Chem 339:52–60
Stark WJ, Maciejewski M, Mädler L, Pratsinis SE, Baiker A (2003) Flame-made nanocrystalline ceria/zirconia: structural properties and dynamic oxygen exchange capacity. J Catal 220:35–43
Ozawa M (1998) Role of cerium-zirconium mixed oxides as catalysts for car pollution: a short review. J Alloys Compd 275–277:886–890
Zoppi MS, Nithiarasu P, Priya NS, Somayaji C, Kanagaraj S (2013) Optimization of ceria-zirconia solid solution based on OSC measurement by cyclic heating process. Proc Eng 64:1235–1241
Taniguchi T, Watanebe T, Ichinohe S, Yoshimura M, Katsumata K, Okada K, Matsushita N (2010) Nanoscale heterogeneities in CeO2–ZrO2 nanocrystals highlighted by UV-resonant Raman spectroscopy. Nanoscale 2:1426–1428
Martínez-Arias A, Fernández-García M, Hungría A-B, Conesa JC, Munuera G (2003) Spectroscopic characterization of heterogeneity and redox effects in zirconium–Cerium (1:1) mixed oxides prepared by microemulsion methods. J Phys Chem B 107:2667–2677
Huang W, Yang J, Wang C, Zou B, Meng X, Wang Y, Cao X, Wang Z (2012) Effects of Zr/Ce molar ratio and water content on thermal stability and structure of ZrO2–CeO2 mixed oxides prepared via sol-gel process. Mater Res Bull 47:2349–2356
Lin S, Yang L, Yang X, Zhou R (2014) Redox behavior of active PdOx species on (Ce, Zr)xO2–Al2O3 mixed oxides and its influence on the three-way catalytic performance. Chem Eng J 247:42–49
Li G, Wang Q, Zhao B, Zhou R (2012) A new insight into the role of transition metals do** with CeO2–ZrO2 and its application in Pd-only three-way catalysts for automotive emission control. Fuel 92:360–368
Zhao B, Wang Q, Li G, Zhou R (2010) Effect of synthesis condition on properties of Ce0.67Zr0.33O2 mixed oxides and its application in Pd-only three-way catalysts. J Alloys Compd 508:500–506
Scholes FH, Soste C, Hughes AE, Hardin SG, Curtis PR (2006) The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl Surf Sci 253:1770–1780
Zhou Y, Lan L, Gong M, Chen Y (2016) Modification of the thermal stability of doped CeO2–ZrO2 mixed oxides with the addition of triethylamine and its application as a Pd-only three-way catalyst. J Mater Sci 51:4283–4295. doi:10.1007/s10853-016-9733-x
Mandal BP, Grover V (2009) Improvement of physico-chemical properties by addition of H2O2: an extensive case study on the RE-doped ceria system (RE = Gd, Sm). J Mater Res 24:2845–2854
Colon G, Navio JA, Monaci R, Ferino I (2000) CeO2–La2O3 catalytic system Part I. Preparation and characterisation of catalysts. Phys Chem Chem Phys 2:4453–4459
Lee J-S, Choi S-C (2004) Crystallization behavior of nano-ceria powders by hydrothermal synthesis using a mixture of H2O2 and NH4OH. Mater Lett 58:390–393
Scholes FH, Hughes AE, Hardin SG, Lynch P, Miller PR (2007) Influence of hydrogen peroxide in the preparation of nanocrystalline ceria. Chem Mater 19:2321–2328
Pappacena A, Porreca P, Boaro M, de Leitenburg C, Trovarelli A (2012) Effect of process modification and presence of H2O2 in the synthesis of samaria-doped ceria powders for fuel cell applications. Int J Hydrogen Energy 37:1698–1709
Navio JA, Colón G, Sánchez-Soto PJ, Macias M (1997) Effects of H2O2 and SO4 2− species on the crystalline structure and surface properties of ZrO2 processed by alkaline precipitation. Chem Mater 9:1256–1261
Huang L, Chen S, Zhu Y, Gong M, Chen Y (2013) Preparation of Ce0.65Zr0.35O2 by co-precipitation: the role of hydrogen peroxide. J Rare Earths 31:461–469
Czapski G, Bielski BHJ, Sutin N (1963) The kinetics of the oxidation of hydrogen peroxide by cerium(IV). J Phys Chem 67(1):201–203
Sugiura M, Ozawa M, Suda A, Suzuki T, Kanazawa T (2005) Development of innovative three-way catalysts containing ceria-circonia solid solutions with high oxygen storage/release capacity. Bull Chem Soc Jpn 78:752–767
Saada R, Kellici S, Heil T, Morgan D, Saha B (2015) Greener synthesis of dimethyl carbonate using a novel ceria-zirconia oxide/graphene nanocomposite catalyst. Appl Catal B Environ 168–169:353–362
Francisco MSP, Mastelaro VR, Nascente PAP, Florentino AO (2001) Activity and characterization by XPS, HR-TEM, Raman spectroscopy, and BET surface area of CuO/CeO2-TiO2 catalysts. J Phys Chem B 105:10515–10522
Lubna RS, Bakhtyar A, Hao Z, Wang WG, Song YQ, Zhang HW, Shah SI, **ao JQ (2009) Detailed study on the role of oxygen vacancies in structural, magnetic and transport behavior of magnetic insulator: Co-CeO2. J Phys Condens Matter 21:1–9
Galtayries A, Sporken R, Riga J, Blanchard G, Caudano R (1998) XPS comparative study of ceria/zirconia mixed oxides: powders and thin film characterisation. J Electron Spectrosc 88:951–956
Reddy BM, Khan A (2005) Nanosized CeO2-SiO2, CeO2-TiO2, and CeO2-ZrO2 mixed oxides: influence of supporting oxide on thermal stability and oxygen storage properties of ceria. Catal Surv Asia 9:155–171
Bensalem A, Muller JC, Bozonverduraz F (1992) From bulk CeO2 to supported cerium–oxygen clusters: a diffuse reflectance approach. J Chem Soc Farady Trans 88:153–154
Bensalem A, Bozon-Verduraz F, Delamar M, Bugli G (1995) Preparation and characterization of highly dispersed silica-supported ceria. Appl Catal A Gen 121:81–93
Reddy BM, Bharali P, Saikia P, Thrimurthulu G, Yamada Y, Kobayashi T (2009) Thermal stability and dispersion behavior of nanostructured CexZr1−xO2 mixed oxides over anatase-TiO2: a combined study of CO oxidation and characterization by XRD, XPS, TPR, HREM, and UV–Vis DRS. Ind Eng Chem Res 48:453–462
Barton DG, Shtein M, Wilson RD, Soled SL, Iglesia E (1999) Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J Phys Chem B 103:630–640
Filtschew A, Hofmann K, Hess C (2016) Ceria and its defect structure: new insights from a combined spectroscopic approach. J Phys Chem C 120:6694–6703
Tsunekawa S, Fukuda T, Kasuya A (2000) Blue shift in ultraviolet absorption spectra of monodisperse CeO2-x nanoparticles. J Appl Phys 87:1318–1321
Si R, Zhang Y-W, Li S-J, Lin B-X, Yan C-H (2004) Urea-based hydrothermally derived homogeneous nanostructured Ce1-xZrxO2 (x = 0–0.8) solid solutions: a strong correlation between oxygen storage capacity and lattice strain. J Phys Chem B 108:12481–12488
Taniguchi T, Watanabe T, Sugiyama N, Subramani AK, Wagata H, Matsushita N, Yoshimura M (2009) Identifying defects in ceria-based nanocrystals by UV resonance Raman spectroscopy. J Phys Chem C 113:19789–19793
Li G, Wang Q, Zhao B, Shen M, Zhou R (2011) Effect of iron do** into CeO2–ZrO2 on the properties and catalytic behaviour of Pd-only three-way catalyst for automotive emission control. J Hazard Mater 186:911–920
Wu Z, Li M, Howe J, Meyer HM, Overbury SH (2010) Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 26:16595–16606
Fornasiero P, Kašpar J, Graziani M (1999) On the rate determining step in the reduction of CeO2-ZrO2 mixed oxides. Appl Catal B Environ 22:L11–L14
Vidal H, Kašpar J, Pijolat M, Colon G, Bernal S, Cordón A, Perrichon V, Fally F (2000) Redox behavior of CeO2-ZrO2 mixed oxides: I. Influence of redox treatments on high surface area catalysts. Appl Catal B Environ 27:49–63
Dong F, Suda A, Tanabe T, Nagai Y, Sobukawa H, Shinjoh H, Sugiura M, Descorme C, Duprez D (2004) Dynamic oxygen mobility and a new insight into the role of Zr atoms in three-way catalysts of Pt/CeO2-ZrO2. Catal Today 93–95:827–832
Fornasiero P, Balducci G, Di Monte R, Kašpar J, Sergo V, Gubitosa G, Ferrero A, Graziani M (1996) Modification of the redox behaviour of CeO2 induced by structural do** with ZrO2. J Catal 164:173–183
Lan L, Chen S, Zhao M, Gong M, Chen Y (2014) The effect of synthesis method on the properties and catalytic performance of Pd/Ce0.5Zr0.5O2-Al2O3 three-way catalyst. J Mol Catal A Chem 394:10–21
Suda A, Sobukawa H, Suzuki T, Kandori T, Ukyo Y, Sugiura M, Kimura M, Hiroshi H, Ikeda Y (1999) Solid solution particle of oxides, a process for producing the same and a catalyst for purifying exhaust gases. U. S. Patent 5,958,827
Maruki M, Okamoto H, Kodama H, Izumi A (2010) Cerium-zirconium based compound oxide and production method thereof. U. S. Patent 7,795,171
Woodhead, J. L (1980) Process for preparing aqueous dispersion of ceria and resulting product. U. S. Patent 4,231,893
Acknowledgements
We gratefully acknowledge the National Key Research and Development Program of China (2016YFC0204901) for the generous financial support to our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, J., Zhou, Y., Cui, Y. et al. The influence of H2O2 on the properties of CeO2-ZrO2 mixed oxides. J Mater Sci 52, 5242–5255 (2017). https://doi.org/10.1007/s10853-017-0765-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0765-7