Abstract
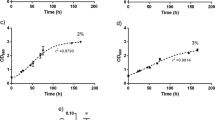
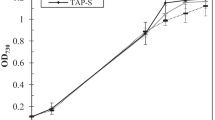
Biohydrogen is an environmentally friendly alternative energy carrier that can be produced by a number of different microorganisms. The unicellular halotolerant cyanobacterium Aphanothece halophytica is one of the high potential H2 producers. Under dark fermentation, it is capable of producing H2 by the bidirectional hydrogenase activity via the catabolism of glycogen stored during photosynthesis. This work aimed to cultivate A. halophytica in natural seawater containing high salinity and minerals, with an addition of some essential nutrients, and to investigate effects of various nutritional and physical factors on its dark fermentative H2 production. A. halophytica was able to grow in natural seawater added with NaNO3. Cells grown in seawater supplemented with as little as 1.76 mM NaNO3 showed similar growth to those cultivated in normal BG11 supplemented with Turk Island salt solution. H2 production was the highest when incubating the cells in seawater without any supplementation of NaNO3. Under this condition, the highest rate of dark fermentative H2 production of 82.79 ± 3.47 nmol H2 mg-1 dry weight h−1 was found in cells incubated at 35 °C, pH 6 with the supplementation of 378 mmolC L−1 glucose, 0.25 M NaCl, and 0.4 μM Fe3+. Long-term H2 accumulation of 1,864 ± 81 nmol H2 mg−1 dry weight was observed after 8 days of dark incubation under anoxic condition, and the high yield of H2 was sustained at least up to 14 days, suggesting the possibility of utilizing natural seawater to grow A. halophytica for long-term production of H2.




Similar content being viewed by others
References
Ahern KS, Ahern CR, Udy JW (2007) Nutrient additions generate prolific growth of Lyngbya majuscula (cyanobacteria) in field and bioassay experiments. Harmful Algae 6:134–151
Allahverdiyeva Y, Leino H, Saari L, Fewer DP, Shunmugam S, Sivonen K, Aro E-M (2010) Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int J Hydrogen Energy 35:1117–1127
Ananyev G, Carrieri D, Dismukes GC (2008) Optimization of metabolic capacity and flux through environmental cues to maximize hydrogen production by the cyanobacterium “Arthrospira (Spirulina) maxima”. Appl Environ Microbiol 74:6102–6113
Baebprasert W, Lindblad P, Incharoensakdi A (2010) Response of H2 production and Hox-hydrogenase activity to external factors in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Int J Hydrogen Energy 35:6611–6616
Benemann JR (2000) Hydrogen production by microalgae. J Appl Phycol 12:291–300
Bothe H, Schmitz O, Yates MG, Newton WE (2010) Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol Mol Biol Rev 74:529–551
Carrieri D, Ananyev G, Garcia Costas AM, Bryant DA, Dismukes GC (2008) Renewable hydrogen production by cyanobacteria: nickel requirements for optimal hydrogenase activity. Int J Hydrogen Energy 33:2014–2022
Dubois M, Gilles KA, Hamilton JK, Reberr PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ernst A, Kirschenlohr H, Diez J, Böger P (1984) Glycogen content and nitrogenase activity in Anabaena variabilis. Arch Microbiol 140:120–125
Garlick S, Oren A, Padan E (1977) Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol 29:623–629
Harrison PJ, Berges JA (2005) Marine culture media. In: Andersen RA (ed) Algal culturing techniques, 1st edn. Elsevier, Amsterdam, pp 21–33
Heyer H, Stal LJ, Krumbein WE (1989) Simultaneous heterolactic and acetate fermentation in the marine cyanobacterium Oscillatoria limosa isolated anaerobically in the dark. Arch Microbiol 151:558–564
Khetkorn W, Khanna N, Incharoensakdi A, Lindblad P (2013) Metabolic and genetic engineering of cyanobacteria for enhanced hydrogen production. Biofuels 4:535–561
Kumaraswamy GK, Guerra T, Qian X, Zhang S, Bryant DA, Dismukes GC (2013) Reprogramming the glycolytic pathway for increased hydrogen production in cyanobacteria: metabolic engineering of NAD+-dependent GAPDH. Energ Environ Sci. doi:10.1039/c3ee42206b
Kumazawa S, Mitsui A (1981) Characterization and optimization of hydrogen photoproduction by a saltwater blue-green alga, Oscillatoria sp. Miami BG7. I. Enhancement through limiting the supply of nitrogen nutrients. Int J Hydrogen Energy 6:339–348
Luo YH, Mitsui A (1994) Hydrogen production from organic substrates in an aerobic nitrogen-fixing marine unicellular cyanobacterium Synechococcus sp. strain Miami BG 043511. J Biotechnol Bioeng 44:1255–1260
MacKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
McNeely K, Xu Y, Bennette N, Bryant DA, Dismukes GC (2010) Redirecting reductant flux into hydrogen production via metabolic engineering of fermentative carbon metabolism in a cyanobacterium. Appl Environ Microbiol 76:5032–5038
Prabaharan D, Subramanian G (1996) Oxygen-free hydrogen production by the marine cyanobacterium Phormidium valderianum BDU 20041. Bioresour Technol 57:111–116
Prabaharan D, Kumar DA, Uma L, Subramanian G (2010) Dark hydrogen production in nitrogen atmosphere—an approach for sustainability by marine cyanobacterium Leptolyngbya valderiana BDU 20041. Int J Hydrogen Energy 35:10725–10730
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Saha SK, Uma L, Subramanian G (2003) Nitrogen stress induced changes in the marine cyanobacterium Oscillatoria willei BDU 130511. FEMS Microbiol Ecol 45:263–272
Taikhao S, Junyapoon S, Incharoensakdi A, Phunpruch S (2013) Factors affecting biohydrogen production by unicellular halotolerant cyanobacterium Aphanothece halophytica. J Appl Phycol 25:575–585
Takabe T, Incharoensakdi A, Arakawa K, Yokota S (1988) CO2 fixation rate and RuBisCO content increase in the halotolerant cyanobacterium, Aphanothece halophytica, grown in high salinities. Plant Physiol 88:1120–1124
Thajuddin N, Subramanian G (2005) Cyanobacterial biodiversity and potential applications in biotechnology. Curr Sci 89:47–57
Tindall DR, Yopp JH, Miller DM, Schmid WE (1978) Physico-chemical parameters governing the growth of Aphanothece halophytica (Chroococcales) in hypersaline media. Phycologia 17:179–185
van der Oost J, Bulthuis BA, Feitz S, Krab K, Kraayenhof R (1989) Fermentation metabolism of the unicellular cyanobacterium Cyanothece PCC 7822. Arch Microbiol 152:415–419
Vignais PM, Billoud B, Meyer J (2001) Classification and phylogeny of hydrogenases. FEMS Microbiol Rev 25:455–501
Wyman M, Gregory RPF, Carr NG (1985) Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science 230:818–820
Yopp JH, Tindall DR, Miller DM, Schmid WE (1978) Isolation, purification and evidence for a halophilic nature of the blue-green alga Aphanothece halophytica Frémy (Chroococcales). Phycol 17:172–178
Acknowledgments
This study was financially supported by research grant from the Faculty of Science, King Mongkut’s Institute of Technology Ladkrabang and the Commission on Higher Education (CHE), Thailand (The university staff development consortium). S. Taikhao is also thankful to the Strategic Scholarships for Frontier Research Network for the Ph.D. program provided by CHE. A. Incharoensakdi thanks the CHE and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University, for the National Research University Project grant (FW0659A), and the Chulalongkorn University Centenary Academic Development Project grant (RES560530052-FW), respectively.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Taikhao, S., Incharoensakdi, A. & Phunpruch, S. Dark fermentative hydrogen production by the unicellular halotolerant cyanobacterium Aphanothece halophytica grown in seawater. J Appl Phycol 27, 187–196 (2015). https://doi.org/10.1007/s10811-014-0292-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0292-8