Abstract
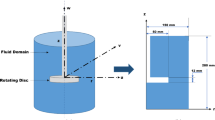
A fully integrated mass transfer and electrochemical model of a lithium production cell solved using a finite element method is presented. The coupled effect of momentum and mass transfer, kinetics, and electric fields is taken all into account. The turbulent flow resulting from the bubbles generated at the anode is solved based on a k-\(\epsilon\) model. The ohmic overpotential and hyperpolarization due to the bubbles are considered through a resistive layer and bubble coverage at the surface of the anode. Furthermore, the effects of the anode–cathode distance (ACD) and current density on the electric and concentration fields of the cell are simulated. The results of the transient simulation reveal that the diaphragm separates the cell in two regions with different flow fields: a first region between the anode and the diaphragm and a second zone between the diaphragm and the cathode. The flow of bubbles generated at the anode has an important impact only in the first region, where the most important gradients are found. The concentration of ions is uniform in the second region. As expected, the current density plays an important role in the electrokinetic of the cell. The change in the geometry of the cell, studied by varying the ACD or by removing the diaphragm, has an important impact on the cell potential and on the concentration of electroactive species near the electrodes. The cell voltage is reduced by as much as 40% when the diaphragm is removed.
Graphical abstract

















Similar content being viewed by others
Abbreviations
- c:
-
Concentration (mol m−3)
- D:
-
Diffusion coefficient (m2 s−1)
- Deff :
-
Effective diffusion coefficient (m2 s−1)
- d:
-
Diameter (m)
- E o :
-
Eotvos number
- F:
-
Faraday‘s constant (A s mol−1)
- g:
-
Earth gravitational acceleration (m s−2)
- i:
-
Current density (A m−2)
- i0 :
-
Exchange current density (A m−2)
- im :
-
Average current density (A m−2)
- k:
-
Turbulent kinetic energy (m2 s−2)
- k r :
-
Reaction rate constant (m s−1)
- M:
-
Morton number
- n:
-
Number of electrons
- N:
-
Mole flux (mol m−2 s−1)
- R:
-
Gas constant (J mol−1 K−1)
- R′:
-
Production term (mol m−3 s−1)
- Re:
-
Reynolds
- T:
-
Time (s)
- T:
-
Temperature (K)
- um :
-
Ions mobility (m2 s−1 V−1)
- u T :
-
Bubble terminal velocity (m s−1)
- V:
-
Velocity vector (m s−1)
- x:
-
Mole fraction
- z:
-
Valence
- α:
-
Transfer coefficient
- \(\epsilon\) :
-
Rate of dissipation of kinetic energy (m2 s−3)
- σ:
-
Conductivity (S m−1)
- ρ:
-
Density (kg m−3)
- μ:
-
Viscosity (kg s−1 m−1)
- γ :
-
Surface tension (N m−1)
- Φ:
-
Potential field (V)
- \(\emptyset _{{\text{g}}}\) :
-
Bubble coverage
- η:
-
Activation overpotential (V)
- a, c:
-
Anode/cathode
- b:
-
Bubble
- l:
-
Electrolyte
- i:
-
Species i
- j:
-
Species j
- m:
-
Average
- mix:
-
Mixture
- s:
-
Electrodes’ surface
- T:
-
Turbulent
- *:
-
Bulk
References
Muller EDJ, Bauer R, Sermond B (1988) Process and apparatus for producing high-purity lithium metal by fused salt electrolysis. US4740279 A
Cooper JF, Krikorian OH, Homsy R V (1979) Electrolytic method for the production of lithium using a lithium-amalgam electrode. US4156635 A
James CD (1924) Electrolytic process and cell. US1501756 A
Verdier JM, Jacubert S, Grosbois J, Dumousseau JY (1986) Continuous electrolysis of lithium chloride into lithium metal. US4617098
Nakamura E, Takata H, Yokoyama Y, Miyamoto H (2010) Process for producing metallic lithium. US20100051470
Le Roux E, Nataf P, Jacubert S (1988) Continuous production of lithium metal by electrolysis of lithium chloride. US4724055 A
Amouzegar K, Harrison S (1996) Electrolytic production of lithium metal. Report, HydroQuebec, Shawinigan
van Vuuren DS, Swanepoel E (2014) Fundamental differences between magnesium and alkali metal electrowinning. Adv Mater Res 1019:160–168
Janz GJ, Bansal NP (1982) Molten salts data: diffusion coefficients in single and multi-component salt systems. J Phys Chem Ref Data 11(3):505
Van Artsdalen ER, Yaffe IS (1955) Electrical conductance and density of molten salt systems: KCl–LiCl, KCl–NaCl and KCl–KI. J Phys Chem 59(2):118–127
Sridharan K, Martin S, Mohammadian M, Sager J, Allen T, Simpson MM, Anderson M, Simpson MM (2012) Thermal properties of LiCl-KCl molten salt for nuclear waste separation. Trans Am Nucl Soc. 106:1240–1241
Andreassen K (1978) Electrolytic production of magnesium. Erzmetall J Explor Min Process Metall Recycl A. 31(7–8):301–309
Taylor R, Krishna R (1993) Multicomponent mass transfer, vol 2. Wiley, New York, p 243
Ariana M, Désilets M, Proulx P (2014) On the analysis of ionic mass transfer in the electrolytic bath of an aluminum reduction cell. Can J Chem Eng 92(11):1951–1964
Bard A, Faulkner L (2001) Electrochemical methods: fundamentals and applications, vol 38, 2nd edn. Wiley, New York, pp 1505–1506
Aldas K, Pehlivanoglu N, Mat MD (2008) Numerical and experimental investigation of two-phase flow in an electrochemical cell. Int J Hydrogen Energy 33(14):3668–3675
Vogt H, Balzer RJ (2005) The bubble coverage of gas-evolving electrodes in stagnant electrolytes. Electrochim Acta 50(3):2073–2079
Liu C-L, Sun Z, Lu G-M, Song X-F, Yu J-G (2015) Experimental and numerical investigation of two-phase flow patterns in magnesium electrolysis cell with non-uniform current density distribution. Can J Chem Eng 93(3):565–579
Clift R, Grace JR, Weber ME (1978) Bubbles, Drops, and particles. Academic Press, New York
Versteeg HK, Malalasekera W (2007) An introduction to computional fluid dynamics: The finite, vol method. Longman Scientific & Technical, New York, p 71
Huber G, Lutz M, Wille M et al (2012) Electrolysis device for the production of alkali metal. US8114258
Bergmann OR, Blank HM, Diemer RB et al (2000) Modified electrolyte and diaphragm for fused salt electrolysis. US6063247A
Acknowledgements
The authors wish to thank Dr. Mohsen Ariana, Postdoctoral researcher at Université de Sherbrooke, for his useful suggestions. We also appreciate Hydro-Québec for their financial support, for providing us with the experimental results and giving us the opportunity to publish this work. The authors are also grateful to the Natural Sciences and Engineering Council of Canada and Canadian (NSERC) and Network for Research and Innovation in Machining Technology for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliaii, E., Désilets, M. & Lantagne, G. Numerical analysis of the effect of structural and operational parameters on electric and concentration fields of a lithium electrolysis cell. J Appl Electrochem 47, 711–726 (2017). https://doi.org/10.1007/s10800-017-1073-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1073-2