Abstract
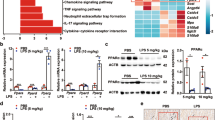
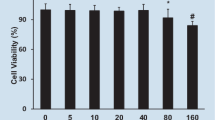
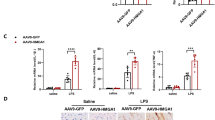
Mitochondrial damage is considered one of the main pathogenetic mechanisms in septic cardiomyopathy. Peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) is critical for maintaining energy homeostasis in different organs and in various physiological and pathological states. It is also a key regulator gene in mitochondrial metabolism. In this study, we investigated whether regulation of the PGC-1α gene had protective effects on septic cardiomyopathy. We developed a rat model of septic cardiomyopathy. H9c2 myocardiocytes were treated with lipopolysaccharide (LPS) and PGC-1α expression measured. PGC-1α-overexpressing lentivirus was used to transfect H9c2 cells. ZLN005 was used to activate PGC-1α. The effect of the inhibition of PGC-1α expression on myocardial cell injury and its underlying mechanisms were also explored. Cell viability was measured by CCK-8 assay. Mitochondrial damage was determined by measuring cellular ATP, reactive oxygen species, and the mitochondrial membrane potential. An apoptosis analysis kit was used to measure cellular apoptosis. Mitochondrial DNA was extracted and real-time PCR performed. LC3B, mitochondrial transcription factor A (TFA), P62, Bcl2, and Bax were determined by immunofluorescence. LC3B, TFA, P62, Parkin, PTEN-induced putative kinase 1, and PGC-1α proteins were determined by Western blotting. We found mitochondrial damage and apoptotic cells in the myocardial tissue of rats with septic cardiomyopathy and in LPS-treated cardiomyocytes. PGC-1α expression was decreased in the late phase of septic cardiomyopathy and in LPS-treated cardiomyocytes. PGC-1α activation by ZLN005 and PGC-1α overexpression reduced apoptosis in myocardiocytes after LPS incubation. PGC-1α gene overexpression alleviated LPS-induced cardiomyocyte mitochondrial damage by activating mitochondrial biogenesis and autophagy functions. Our study indicated that mitochondrial damage and apoptosis occurred in septic cardiomyopathy and LPS-treated cardiomyocytes. The low expression level of PGC-1α protein may have contributed to this damage. By activating the expression of PGC-1α, apoptosis was reduced in cardiomyocytes. The underlying mechanism may be that PGC-1α can activate mitochondrial biogenesis and autophagy functions, reducing mitochondrial damage and thereby reducing apoptosis.




Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- HE:
-
Hematoxylin-eosin staining
- RT-PCR:
-
Real-time polymerase chain reaction
- MAP:
-
Mean artery pressure
- LVEDP:
-
Left ventricular end-diastolic pressure
- PGC-1α:
-
Peroxisome proliferator activated receptor gamma coactivator 1 alpha
- ROS:
-
Reactive oxygen species
- mtDNA:
-
Mitochondrial DNA
- PINK1:
-
PTEN-induced putative kinase 1
- mt-Tfam:
-
Mitochondrial expression of transcription factors
References
Jonckheere, A.I., J.A. Smeitink, and R.J. Rodenburg. 2012. Mitochondrial ATP synthase: architecture, function and pathology. Journal of Inherited Metabolic Disease 35: 211–225.
Narendra, D.P., and R.J. Youle. 2011. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxidants & Redox Signaling 14: 1929–1938.
Koene, S., and J. Smeitink. 2011. Metabolic manipulators: a well founded strategy to combat mitochondrial dysfunction. Journal of Inherited Metabolic Disease 34: 315–325.
Chistiakov, D.A., T.P. Shkurat, A.A. Melnichenko, A.V. Grechko, and A.N. Orekhov. 2018. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Annals of Medicine 50: 121–127.
Cimolai, M.C., S. Alvarez, C. Bode, and H. Bugger. 2015. Mitochondrial mechanisms in septic cardiomyopathy. International Journal of Molecular Sciences 16: 17763–17778.
Disatnik, M.H., S. Hwang, J.C. Ferreira, and D. Mochly-Rosen. 2015. New therapeutics to modulate mitochondrial dynamics and mitophagy in cardiac diseases. Journal of Molecular Medicine 93: 279–287.
Li, S., Q. Yu, G.X. Wang, and J.D. Lin. 2013. The biological clock is regulated by adrenergic signaling in brown fat but is dispensable for cold-induced thermogenesis. PLoS One 8: e70109.
Festuccia, W.T., P.G. Blanchard, T.B. Oliveira, J. Magdalon, V.A. Paschoal, D. Richard, and Y. Deshaies. 2012. PPARgamma activation attenuates cold-induced upregulation of thyroid status and brown adipose tissue PGC-1alpha and D2. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 303: R1277–R1285.
Rowe, G.C., A. Jiang, and Z. Arany. 2010. PGC-1 coactivators in cardiac development and disease. Circulation Research 107: 825–838.
Cheng, C.F., H.C. Ku, and H. Lin. 2018. PGC-1alpha as a pivotal factor in lipid and metabolic regulation. International Journal of Molecular Sciences 19.
Ventura-Clapier, R., A. Garnier, and V. Veksler. 2008. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovascular Research 79(2): 208–217.
Patten, I.S., and Z. Arany. 2012. PGC-1 coactivators in the cardiovascular system. Trends in Endocrinology and Metabolism 23: 90–97.
Settembre, C., C. Di Malta, V.A. Polito, A.M. Garcia, F. Vetrini, S. Erdin, S.U. Erdin, T. Huynh, D. Medina, P. Colella, et al. 2011. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–1433.
Kim, H.K., I.S. Song, S.Y. Lee, S.H. Jeong, S.R. Lee, H.J. Heo, V.T. Thu, N. Kim, K.S. Ko, B.D. Rhee, D.H. Jeong, Y.N. Kim, and J. Han. 2014. B7-H4 downregulation induces mitochondrial dysfunction and enhances doxorubicin sensitivity via the cAMP/CREB/PGC1-alpha signaling pathway in HeLa cells. Pflügers Archiv 466: 2323–2338.
Koka, S., H.S. Aluri, L. **, E.J. Lesnefsky, and R.C. Kukreja. 2014. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. American Journal of Physiology. Heart and Circulatory Physiology 306: H1558–H1568.
Lai, L., M. Wang, O.J. Martin, T.C. Leone, R.B. Vega, X. Han, and D.P. Kelly. 2014. A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. The Journal of Biological Chemistry 289: 2250–2259.
**, Z., L.F. Zhang, Y.J. Cui, Y.M. Chang, C.W. Jiang, Z.Z. Meng, P. Xu, H.Y. Liu, D.Y. Wang, and X.B. Cao. 2015. The protective effects of salidroside from exhaustive exercise-induced heart injury by enhancing the PGC-1 alpha -NRF1/NRF2 pathway and mitochondrial respiratory function in rats. Oxidative Medicine and Cellular Longevity 2015: 876825.
Carre, J.E., J.C. Orban, L. Re, K. Felsmann, W. Iffert, M. Bauer, H.B. Suliman, C.A. Piantadosi, T.M. Mayhew, P. Breen, et al. 2010. Survival in critical illness is associated with early activation of mitochondrial biogenesis. American Journal of Respiratory and Critical Care Medicine 182: 745–751.
Kim, H.J., K.G. Park, E.K. Yoo, Y.H. Kim, Y.N. Kim, H.S. Kim, H.T. Kim, J.Y. Park, K.U. Lee, W.G. Jang, J.G. Kim, B.W. Kim, and I.K. Lee. 2007. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxidants & Redox Signaling 9: 301–307.
Wegner, A., D. Pavlovic, S. Haussmann-Vopel, and C. Lehmann. 2018. Impact of lipid modulation on the intestinal microcirculation in experimental sepsis. Microvascular Research 120: 41–46.
Tang, G., H. Yang, J. Chen, M. Shi, L. Ge, X. Ge, and G. Zhu. 2017. Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget 8: 97977–97989.
Vaez, H., M. Rameshrad, M. Najafi, J. Barar, A. Barzegari, and A. Garjani. 2016. Cardioprotective effect of metformin in lipopolysaccharide-induced sepsis via suppression of toll-like receptor 4 (TLR4) in heart. European Journal of Pharmacology 772: 115–123.
Yang, N., X.L. Shi, B.L. Zhang, J. Rong, T.N. Zhang, W. Xu, and C.F. Liu. 2018. The trend of beta3-adrenergic receptor in the development of septic myocardial depression: a lipopolysaccharide-induced rat septic shock model. Cardiology 139: 234–244.
Zhang, T.N., N. Yang, J.E. Goodwin, K. Mahrer, D. Li, J. **a, R. Wen, H. Zhou, T. Zhang, W.L. Song, and C.F. Liu. 2019. Characterization of circular RNA and microRNA profiles in septic myocardial depression: a lipopolysaccharide-induced rat septic shock model. Inflammation 42: 1990–2002.
Zhang, T.N., J.E. Goodwin, B. Liu, D. Li, R. Wen, N. Yang, J. **a, H. Zhou, T. Zhang, W.L. Song, and C.F. Liu. 2019. Characterization of long noncoding RNA and mRNA profiles in sepsis-induced myocardial depression. Molecular Therapy--Nucleic Acids 17: 852–866.
Arulkumaran, N., C.S. Deutschman, M.R. Pinsky, B. Zuckerbraun, P.T. Schumacker, H. Gomez, A. Gomez, P. Murray, and J.A. Kellum. 2016. Mitochondrial function in sepsis. Shock 45: 271–281.
Pan, P., X. Wang, and D. Liu. 2018. The potential mechanism of mitochondrial dysfunction in septic cardiomyopathy. The Journal of International Medical Research 46: 2157–2169.
Singer, M. 2014. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5: 66–72.
Ho, J., J. Yu, S.H. Wong, L. Zhang, X. Liu, W.T. Wong, C.C. Leung, G. Choi, M.H. Wang, T. Gin, et al. 2016. Autophagy in sepsis: degradation into exhaustion? Autophagy 12: 1073–1082.
Maurer, K., T. Reyes-Robles, F.R. Alonzo, J. Durbin, V.J. Torres, and K. Cadwell. 2015. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host & Microbe 17: 429–440.
Laval, J., A. Singh, and D. Hartl. 2017. Autophagy traps neutrophils into a protective alliance during sepsis. American Journal of Respiratory and Critical Care Medicine 196: 537–538.
Sun, Y., X. Yao, Q.J. Zhang, M. Zhu, Z.P. Liu, B. Ci, Y. **e, D. Carlson, B.A. Rothermel, Y. Sun, B. Levine, J.A. Hill, S.E. Wolf, J.P. Minei, and Q.S. Zang. 2018. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 138: 2247–2262.
Chung, K.W., K.M. Kim, Y.J. Choi, H.J. An, B. Lee, D.H. Kim, E.K. Lee, E. Im, J. Lee, D.S. Im, B.P. Yu, and H.Y. Chung. 2017. The critical role played by endotoxin-induced liver autophagy in the maintenance of lipid metabolism during sepsis. Autophagy 13: 1113–1129.
Funding
This study’s reagents cost was supported by the National Natural Science Foundation of China (No. 81971810). This study’s animals cost was supported by the Natural Science Foundation of Liaoning Province (No. 2017225003, No. 2018108001). This study’s experimental apparatus cost was supported by the Science and Technology Foundation of Shenyang (No. F13-220-9-38) and 345 Talent Project of Sheng**g Hospital of China Medical University.
Author information
Authors and Affiliations
Contributions
T.Z. and C.F.L. conceived and designed the study. T.Z. performed the most assays. T.Z., R.W., and W.L.S analyzed the data. T.Z., T.N.Z, and C.F.L wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The study was approved by the Ethics Committee of Sheng**g Hospital of China Medical University(2018PS121k).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, T., Liu, CF., Zhang, TN. et al. Overexpression of Peroxisome Proliferator-Activated Receptor γ Coactivator 1-α Protects Cardiomyocytes from Lipopolysaccharide-Induced Mitochondrial Damage and Apoptosis. Inflammation 43, 1806–1820 (2020). https://doi.org/10.1007/s10753-020-01255-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01255-4