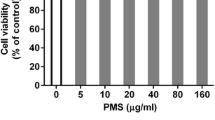
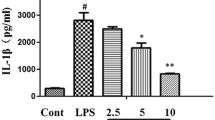
Abstract
Apigenin is one of the plant flavonoids present in fruits and vegetables, acting as an important nutraceutical component. It is recognized as a potential antioxidant, antimicrobial, and anti-inflammatory molecule. In the present study, the mechanism of anti-inflammatory action of apigenin on lipopolysaccharide (LPS)-induced pro-inflammatory cytokines and activator protein-1 (AP-1) factors in human lung A549 cells was investigated. The anti‐inflammatory activity of apigenin on LPS-induced inflammation was determined by analyzing the expression of pro-inflammatory cytokines, nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and different AP-1 factors. Apigenin significantly inhibited the LPS-induced expression of iNOS, COX-2, expression of pro-inflammatory cytokines (IL-1β, IL-2, IL-6, IL-8, and TNF-α), and AP-1 proteins (c-Jun, c-Fos, and JunB) including nitric oxide production. Study confirms the anti-inflammatory effect of apigenin by inhibiting the expression of inflammatory mediators and AP-1 factors involved in the inflammation and its importance in the treatment of lung inflammatory diseases.







Similar content being viewed by others
REFERENCES
Hanada, T., and A. Yoshimura. 2002. Regulation of cytokine signaling and inflammation. Cytokine & Growth Factor Reviews 13(4–5): 413–421.
Vincent, J.L., Q. Sun, and M.J. Dubois. 2002. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America 34(8): 1084–1093. doi:10.1086/339549.
Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, et al. 1997. Bacterial DNA causes septic shock. Nature 386(6623): 336–337. doi:10.1038/386336a0.
Karima, R., S. Matsumoto, H. Higashi, and K. Matsushima. 1999. The molecular pathogenesis of endotoxic shock and organ failure. Molecular Medicine Today 5(3): 123–132. doi:10.1016/S1357-4310(98)01430-0.
Tobias, P.S., R.I. Tap**, and J.A. Gegner. 1999. Endotoxin interactions with lipopolysaccharide-responsive cells. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America 28(3): 476–481. doi:10.1086/515163.
Xagorari, A., A. Papapetropoulos, A. Mauromatis, M. Economou, T. Fotsis, and C. Roussos. 2001. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. The Journal of Pharmacology and Experimental Therapeutics 296(1): 181–187.
Wang, H.Q., and R.C. Smart. 1999. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. Journal of Cell Science 112(Pt 20): 3497–3506.
Lantz, R.C., G.J. Chen, A.M. Solyom, S.D. Jolad, and B.N. Timmermann. 2005. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 12(6–7): 445–452. doi:10.1016/j.phymed.2003.12.011.
Tracey, K.J., Y. Fong, D.G. Hesse, K.R. Manogue, A.T. Lee, G.C. Kuo, et al. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330(6149): 662–664. doi:10.1038/330662a0.
Dinarello, C.A. 1997. Interleukin-1. Cytokine & Growth Factor Reviews 8(4): 253–265.
Papavassiliou, A.G. 1995. Transcription factors: structure, function, and implication in malignant growth. Anticancer Research 15(3): 891–894.
Shaulian, E., and M. Karin. 2002. AP-1 as a regulator of cell life and death. Nature Cell Biology 4(5): E131–E136. doi:10.1038/ncb0502-e131.
Chinenov, Y., and T.K. Kerppola. 2001. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20(19): 2438–2452. doi:10.1038/sj.onc.1204385.
Stafford, H.A. 1992. Flavonoid metabolism. Boca Raton: CRC Press.
Middleton, E. 1996. The flavonoids as potential therapeutic agents. In Immunopharmaceuticals, ed. E.S. Kimball, 227–257. Boca Raton: CRC Press.
Babu, R.L., M. Naveen Kumar, R.H. Patil, K.S. Devaraju, G.T. Ramesh, and S.C. Sharma. 2013. Effect of estrogen and tamoxifen on the expression pattern of AP-1 factors in MCF-7 cells: role of c-Jun, c-Fos, and Fra-1 in cell cycle regulation. Molecular and Cellular Biochemistry 380(1–2): 143–151. doi:10.1007/s11010-013-1667-x.
Periyakaruppan, A., F. Kumar, S. Sarkar, C.S. Sharma, and G.T. Ramesh. 2007. Uranium induces oxidative stress in lung epithelial cells. Archives of Toxicology 81(6): 389–395. doi:10.1007/s00204-006-0167-0.
Eigler, A., J. Moeller, and S. Endres. 1995. Exogenous and endogenous nitric oxide attenuates tumor necrosis factor synthesis in the murine macrophage cell line RAW 264.7. Journal of Immunology (Baltimore, Md: 1950) 154(8): 4048–4054.
Sharma, S.C., J.W. Clemens, M.D. Pisarska, and J.S. Richards. 1999. Expression and function of estrogen receptor subtypes in granulosa cells: regulation by estradiol and forskolin. Endocrinology 140(9): 4320–4334. doi:10.1210/endo.140.9.6965.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254.
Murakami, A., and H. Ohigashi. 2007. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. International Journal of Cancer Journal International du Cancer 121(11): 2357–2363. doi:10.1002/ijc.23161.
Levine, S.J. 1995. Bronchial epithelial cell-cytokine interactions in airway inflammation. Journal of Investigative Medicine: the Official Publication of the American Federation for Clinical Research 43(3): 241–249.
Barnes, P.J., and I. Adcock. 1993. Anti-inflammatory actions of steroids: molecular mechanisms. Trends in Pharmacological Sciences 14(12): 436–441.
Vane, S.J. 1998. Differential inhibition of cyclooxygenase isoforms: an explanation of the action of NSAIDs. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases 4(5 Suppl): s3–s10.
Iwashita, K., M. Kobori, K. Yamaki, and T. Tsushida. 2000. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Bioscience, Biotechnology, and Biochemistry 64(9): 1813–1820. doi:10.1271/bbb.64.1813.
Mak, P., Y.K. Leung, W.Y. Tang, C. Harwood, and S.M. Ho. 2006. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia 8(11): 896–904. doi:10.1593/neo.06538.
Liang, Y.C., Y.T. Huang, S.H. Tsai, S.Y. Lin-Shiau, C.F. Chen, and J.K. Lin. 1999. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 20(10): 1945–1952.
Raso, G.M., R. Meli, G. Di Carlo, M. Pacilio, and R. Di Carlo. 2001. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sciences 68(8): 921–931.
Jeong, G.S., S.H. Lee, S.N. Jeong, Y.C. Kim, and E.C. Kim. 2009. Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. International Immunopharmacology 9(12): 1374–1380. doi:10.1016/j.intimp.2009.08.015.
Murakami, A., D. Takahashi, K. Hagihara, K. Koshimizu, and H. Ohigashi. 2003. Combinatorial effects of nonsteroidal anti-inflammatory drugs and food constituents on production of prostaglandin E2 and tumor necrosis factor-alpha in RAW264.7 murine macrophages. Bioscience, Biotechnology, and Biochemistry 67(5): 1056–1062.
Boucher, J.L., C. Moali, and J.P. Tenu. 1999. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cellular and Molecular Life Sciences: CMLS 55(8–9): 1015–1028.
Patil, R.H., R.L. Babu, M. Naveen Kumar, K.M. Kiran Kumar, S.M. Hegde, G.T. Ramesh, et al. 2015. Apigenin inhibits PMA-induced expression of pro-inflammatory cytokines and AP-1 factors in A549 cells. Molecular and Cellular Biochemistry 403(1–2): 95–106. doi:10.1007/s11010-015-2340-3.
Chung, W.Y., J.H. Park, M.J. Kim, H.O. Kim, J.K. Hwang, S.K. Lee, et al. 2007. Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-kappa B. Carcinogenesis 28(6): 1224–1231. doi:10.1093/carcin/bgm005.
Andreakos, E., B. Foxwell, and M. Feldmann. 2004. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunological Reviews 202: 250–265. doi:10.1111/j.0105-2896.2004.00202.x.
Jachak, S.M. 2006. Cyclooxygenase inhibitory natural products: current status. Current Medicinal Chemistry 13(6): 659–678.
Krakauer, T. 2004. Molecular therapeutic targets in inflammation: cyclooxygenase and NF-kappaB. Current Drug Targets. Inflammation and Allergy 3(3): 317–324.
Verma, N., S.K. Tripathi, D. Sahu, H.R. Das, and R.H. Das. 2010. Evaluation of inhibitory activities of plant extracts on production of LPS-stimulated pro-inflammatory mediators in J774 murine macrophages. Molecular and Cellular Biochemistry 336(1–2): 127–135. doi:10.1007/s11010-009-0263-6.
Mazzucchelli, L., C. Hauser, K. Zgraggen, H. Wagner, M. Hess, J.A. Laissue, et al. 1994. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. The American Journal of Pathology 144(5): 997–1007.
Ohshima, H., and H. Bartsch. 1994. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutation Research 305(2): 253–264.
Johnston, L.C., X. Su, K. Maguire-Zeiss, K. Horovitz, I. Ankoudinova, D. Guschin, et al. 2008. Human interleukin-10 gene transfer is protective in a rat model of Parkinson’s disease. Molecular Therapy: the Journal of the American Society of Gene Therapy 16(8): 1392–1399. doi:10.1038/mt.2008.113.
Arenzana-Seisdedos, F., J. Thompson, M.S. Rodriguez, F. Bachelerie, D. Thomas, and R.T. Hay. 1995. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Molecular and Cellular Biology 15(5): 2689–2696.
Yoon, J.H., and S.J. Baek. 2005. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Medical Journal 46(5): 585–596.
Azzolina, A., A. Bongiovanni, and N. Lampiasi. 2003. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochimica et Biophysica Acta 1643(1–3): 75–83.
Lewis, T.S., P.S. Shapiro, and N.G. Ahn. 1998. Signal transduction through MAP kinase cascades. Advances in Cancer Research 74: 49–139.
Acknowledgments
The authors wish to express their gratitude to the University Grant Commission-Centre with Potential for Excellence in Particular Area (UGC-CPEPA) [8-2/2008(NS/PE)] and the Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE), New Delhi (SR/59/Z-23/2010/38 (c)), for providing financial support. The authors also wish to thank the University Grant Commission- Basic Scientific Research (UGC-BSR), New Delhi, for providing fellowship to RHP and the Department of Microbiology and Biotechnology, Bangalore University, Bengaluru, for providing the facility.
Conflict of Interest
Authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, R.H., Babu, R.L., Naveen Kumar, M. et al. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation 39, 138–147 (2016). https://doi.org/10.1007/s10753-015-0232-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0232-z