Abstract
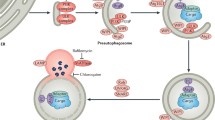
Anti-apoptotic therapy for cardiomyocytes could be an effective strategy for preventing or treating heart failure. Notably, however, morphological evidence definitively demonstrating cardiomyocyte apoptosis has been very rare in actual heart diseases such as acute myocardial infarction and heart failure. By contrast, within the postinfarction heart, interstitial noncardiomyocytes such as granulation tissue cells do die via apoptosis to form scar tissue. Blockade of this apoptosis improves survival and mitigates ventricular remodeling and dysfunction during the chronic stage. Possible mechanisms to explain this benefit might be preservation of infarcted wall thickness and preservation of myofibroblasts, which could promote infarct shrinkage; both would reduce wall stress through Laplace’s law. On the other hand, autophagy is an intracellular degradation mechanism that compensates for energy insufficiency by digesting and recycling intracellular components, and is often observed in cardiomyocytes within failing hearts with various origins including postinfarction. Starvation strongly induces and activates autophagic degeneration within cardiomyocytes. When that activation is inhibited, the starved animals suffer from heart failure. Promoting autophagy through caloric restriction or several reagents not only reduces the acute infarct size but also mitigates postinfarction cardiac remodeling and dysfunction during chronic stages. Moreover, augmenting autophagy by the treatment with resveratrol or exercise can bring about reverse remodeling in failing hearts with a large old myocardial infarction. In conclusion, we propose two strategies for managing postinfarction heart failure through control of cell death/degeneration: (1) anti-apoptosis in granulation tissue noncardiomyocytes; and (2) pro-autophagy in salvaged cardiomyocytes.








Similar content being viewed by others
References
Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G (2005) Nomenclature Committee on Cell Death. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 12(Suppl 2):1463–1467
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G (2009) Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11
Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G (2007) Cell death modalities: classification and pathophysiological implications. Cell Death Differ 14:1237–1243
Takemura G, Kanoh M, Minatoguchi S, Fujiwara H (2013) Cardiomyocyte apoptosis in the failing heart—a critical review from definition and classification of cell death. Int J Cardiol 167:2373–2386
Goldenthal MJ (2016) Mitochondrial involvement in myocyte death and heart failure. Heart Fail Rev 21:137–155
Moe GW, Marín-García J (2016) Role of cell death in the progression of heart failure. Heart Fail Rev 21:157–167
Adameova A, Goncalvesova E, Szobi A, Dhalla NS (2016) Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev 21:213–221
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146:3–15
Takemura G, Kato S, Aoyama T, Hayakawa Y, Kanoh M, Maruyama R, Arai M, Nishigaki K, Minatoguchi S, Fukuda K, Fujiwara T, Fujiwara H (2001) Characterization of ultrastructure and its relation with DNA fragmentation in Fas-induced apoptosis of cultured cardiac myocytes. J Pathol 193:546–556
Maruyama R, Takemura G, Aoyama T, Hayakawa K, Koda M, Kawase Y, Qiu X, Ohno Y, Minatoguchi S, Miyata K, Fujiwara T, Fujiwara H (2001) Dynamic process of apoptosis in adult rat cardiomyocytes analyzed using 48-hour videomicroscopy and electron microscopy: beating and rate are associated with the apoptotic process. Am J Pathol 159:683–691
Hayakawa K, Takemura G, Koda M, Kawase Y, Maruyama R, Li Y, Maruyama R, Li Y, Minatoguchi S, Fujiwara T, Fujiwara H (2002) Sensitivity to apoptosis signal, clearance rate, and ultrastructure of Fas ligand-induced apoptosis in in vivo adult cardiac cells. Circulation 105:3039–3045
Nagata S (1997) Apoptosis by death factor. Cell 88:355–365
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7. https://doi.org/10.1101/cshperspect.a006080
Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335:1182–1189
Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P (1997) Apoptosis in the failing human heart. N Engl J Med 336:1131–1141
Narula J, Kolodgie FD, Virmani R (2000) Apoptosis and cardiomyopathy. Curr Opin Cardiol 15:183–188
Grazette LP, Rosenzweig A (2005) Role of apoptosis in heart failure. Heart Fail Clin 1:251–261
Abbate A, Biondi-Zoccai GG, Baldi A (2002) Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 193:145–153
Hein S, Amon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J (2003) Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 107:984–991
Zhang Z, Vezza R, Plappert T, McNamara P, Lawson JA, Austin S, Praticò D, Sutton MS, FitzGerald GA (2003) COX-2-dependent cardiac failure in Gh/tTG transgenic mice. Circ Res 92:1153–1561
Sano M, Wang SC, Shirai M, Scaglia F, **e M, Sakai S, Tanaka T, Kulkarni PA, Barger PM, Youker KA, Taffet GE, Hamamori Y, Michael LH, Craigen WJ, Schneider MD (2004) Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. EMBO J 23:3559–3569
Engel D, Peshock R, Armstong RC, Sivasubramanian N, Mann DL (2004) Cardiac myocyte apoptosis provokes adverse cardiac remodeling in transgenic mice with targeted TNF overexpression. Am J Physiol Heart Circ Physiol 287:H1303–H1311
Ricci R, Eriksson U, Oudit GY, Eferl R, Akhmedov A, Sumara I, Sumara G, Kassiri Z, David JP, Bakiri L, Sasse B, Idarraga MH, Rath M, Kurz D, Theussl HC, Perriard JC, Backx P, Penninger JM, Wagner EF (2005) Distinct functions of junD in cardiac hypertrophy and heart failure. Genes Dev 19:208–213
Toko H, Oka T, Zou Y, Sakamoto M, Mizukami M, Sano M, Yamamoto R, Sugaya T, Komuro I (2002) Angiotensin II type 1a receptor mediates doxorubicin-induced cardiomyopathy. Hypertens Res 25:597–603
Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr, Chien KR, Lee KF (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8:459–465
Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH (2007) Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370:2011–2019
Borisov AB, Ushakov AV, Zagorulko AK, Novikov NY, Selivanova KF, Edwards CA, Russell MW (2008) Intracardiac lipid accumulation, lipoatrophy of muscle cells and expansion of myocardial infarction in type 2 diabetic patients. Micron 39:944–951
Kostin S, Pool L, Elsässer A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klövekorn WP, Schaper J (2003) Myocytes die by multiple mechanisms in failing human hearts. Circ Res 92:715–724
Liu JJ, Peng L, Bradley CJ, Zulli A, Shen J, Buxton BF (2000) Increased apoptosis in the heart of genetic hypertension, associated with increased fibroblasts. Cardiovasc Res 45:729–735
Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, de Rienzo A, Roncarati R, Trimarco B, Lembo G (1999) Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 99:3071–3078
Walther T, Tschöpe C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, Nikolic A, Wang Y, Ebermann L, Siems WE, Bader M, Shakibaei M, Schultheiss HP, Dörner A (2007) Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation 115:333–344
Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW 2nd, Armstrong RC, Kitsis RN (2003) Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation 108:3036–3041
Levkau B, Schäfers M, Wohlschlaeger J, Westermann D, Heringer-Walther S, Riad A, Nikolic A, Wang Y, Ebermann L, Siems WE, Bader M, Shakibaei M, Schultheiss HP, Dörner A (2008) Survivin determines cardiac function by controlling total cardiomyocyte number. Circulation 117:1583–1593
Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P (1999) Myocyte death in the failing human heart is gender dependent. Circ Res 85:856–866
Abbate A, De Falco M, Morales C, Gelpi RJ, Prisco M, De Luca A, Palleiro J, Fedele V, Feroce F, Baldi F, Vetrovec GW, Baldi A (2007) Electron microscopy characterization of cardiomyocyte apoptosis in ischemic heart disease. Int J Cardiol 114:118–120
Prech M, Marszałek A, Schröder J, Filas V, Lesiak M, Jemielity M, Araszkiewicz A, Grajek S (2010) Apoptosis as a mechanism for the elimination of cardiomyocytes after acute myocardial infarction. Am J Cardiol 105:1240–1245
Sanchis D, Llovera M, Ballester M, Comella JX (2008) An alternative view of apoptosis in heart development and disease. Cardiovasc Res 77:448–451
Inserte J, Cardona M, Poncelas-Nozal M, Hernando V, Vilardosa Ú, Aluja D, Parra VM, Sanchis D, Garcia-Dorado D (2016) Studies on the role of apoptosis after transient myocardial ischemia: genetic deletion of the executioner caspases-3 and -7 does not limit infarct size and ventricular remodeling. Basic Res Cardiol 111:18
Park M, Shen YT, Gaussin V, Heyndrickx GR, Bartunek J, Resuello RR, Natividad FF, Kitsis RN, Vatner DE, Vatner SF (2009) Apoptosis predominates in nonmyocytes in heart failure. Am J Physiol Heart Circ Physiol 297:H785–H791
Jose Corbalan J, Vatner DE, Vatner SF (2016) Myocardial apoptosis in heart disease: does the emperor have clothes? Basic Res Cardiol 111:31
Ohno M, Takemura G, Ohno A, Misao J, Hayakawa Y, Minatoguchi S, Fujiwara T, Fujiwara H (1998) “Apoptotic” myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with in situ nick end-labeling. Circulation 98:1422–1430
Buja LM, Entman ML (1998) Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation 98:1355–1357
Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, Noda T, Fujiwara T, Fukuda K, Minatoguchi S, Fujiwara H (1999) Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: not apoptosis but DNA repair. Circulation 99:2757–2764
Koda M, Takemura G, Kanoh M, Hayakawa K, Kawase Y, Maruyama R, Li Y, Minatoguchi S, Fujiwara T, Fujiwara H (2003) Myocytes positive for in situ markers for DNA breaks in human hearts which are hypertrophic, but neither failed nor dilated: a manifestation of cardiac hypertrophy rather than failure. J Pathol 199:229–236
Takemura G, Fujiwara H (2006) Morphological aspects of apoptosis in heart disease. J Cell Mol Med 10:56–75
Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM (2001) Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res 51:304–312
Bartunek J, Vanderheyden M, Knaapen MW, Tack W, Kockx MM, Goethals M (2002) Deoxyribonucleic acid damage/repair proteins are elevated in the failing human myocardium due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40:1097–1103
White P, Mallory G, Salcedo-Salga J (1936) The speed of healing of myocardial infarcts. Trans Am Clin Climatol Assoc 52:97–104
Fishbein MC, Maclean D, Maroko PR (1978) Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol 90:57–70
Katwa LC, Campbell SE, Tyagi SC, Lee SJ, Cicila GT, Weber KT (1977) Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol 29:1375–1386
Desmoulière A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
Takemura G, Ohno M, Hayakawa Y, Misao J, Kanoh M, Ohno A, Uno Y, Minatoguchi S, Fujiwara T, Fujiwara H (1998) Role of apoptosis in the disappearance of infiltrated and proliferated interstitial cells after myocardial infarction. Circ Res 82:1130–1138
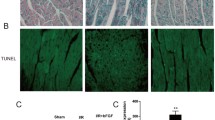
Hayakawa K, Takemura G, Kanoh M, Li Y, Koda M, Kawase Y, Maruyama R, Okada H, Minatoguchi S, Fujiwara T, Fujiwara H (2003) Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation 108:104–109
Li Y, Takemura G, Kosai K, Takahashi T, Okada H, Miyata S, Yuge K, Nagano S, Esaki M, Khai NC, Goto K, Mikami A, Maruyama R, Minatoguchi S, Fujiwara T, Fujiwara H (2004) Critical roles for the Fas/Fas ligand system in postinfarction ventricular remodeling and heart failure. Circ Res 95:627–636
Kanamori H, Takemura G, Li Y, Okada H, Okada H, Maruyama R, Aoyama T, Miyata S, Esaki M, Ogino A, Nakagawa M, Ushikoshi H, Kawasaki M, Minatoguchi S, Fujiwara H (2007) Inhibition of Fas-associated apoptosis in granulation tissue cells accompanies attenuation of postinfarction left ventricular remodeling by olmesartan. Am J Physiol Heart Circ Physiol 292:H2184–H2194
Ogino A, Takemura G, Kanamori H, Okada H, Maruyama R, Miyata S, Esaki M, Nakagawa M, Aoyama T, Ushikoshi H, Kawasaki M, Minatoguchi S, Fujiwara T, Fujiwara H (2007) Amlodipine inhibits granulation tissue cell apoptosis through reducing calcineurin activity to attenuate postinfarction cardiac remodeling. Am J Physiol Heart Circ Physiol 293:H2271–H2280
Reimer KA, Vander Heide RS, Richard VJ (1993) Reperfusion in acute myocardial infarction: effect of timing and modulating factors in experimental models. Am J Cardiol 72:13G–21G
Kim CB, Braunwald E (1993) Potential benefits of late reperfusion of infarcted myocardium. The open artery hypothesis. Circulation 88:2426–2436
Nakagawa M, Takemura G, Kanamori H, Goto K, Maruyama R, Tsujimoto A, Ohno T, Okada H, Ogino A, Esaki M, Miyata S, Li L, Ushikoshi H, Aoyama T, Kawasaki M, Nagashima K, Fujiwara T, Minatoguchi S, Fujiwara H (2008) Mechanisms by which late coronary reperfusion mitigates postinfarction cardiac remodeling. Circ Res 103:98–106
von Harsdorf R (2004) “Fas-ten” your seat belt: anti-apoptotic treatment in heart failure takes off. Circ Res 95:554–556
Takemura G, Nakagawa M, Kanamori H, Minatoguchi S, Fujiwara H (2009) Benefits of reperfusion beyond infarct size limitation. Cardiovasc Res 83:269–276
Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK (2010) Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res 86:103–112
Sala-Mercado JA, Wider J, Undyala VV, Jahania S, Yoo W, Mentzer RM Jr, Gottlieb RA, Przyklenk K (2010) Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation 122:S179–S184
Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK (2009) Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med 13:373–387
Yan L, Sadoshima J, Vatner DE, Vatner SF (2009) Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy 5:709–712
Maeda H, Nagai H, Takemura G, Shintani-Ishida K, Komatsu M, Ogura S, Aki T, Shirai M, Kuwahira I, Yoshida K (2013) Intermittent-hypoxia induced autophagy attenuates contractile dysfunction and myocardial injury in rat heart. Biochim Biophys Acta 1832:1159–1166
Di R, Wu X, Chang Z, Zhao X, Feng Q, Lu S, Luan Q, Hemmings BA, Li X, Yang Z (2012) S6K inhibition renders cardiac protection against myocardial infarction through PDK1 phosphorylation of Akt. Biochem J 441:199–207
Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Kawasaki M, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S (2011) Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol 300:H2261–H2271
Shiomi M, Miyamae M, Takemura G, Kaneda K, Inamura Y, Onishi A, Koshinuma S, Momota Y, Minami T, Figueredo VM (2014) Sevoflurane induces cardioprotection through reactive oxygen species-mediated upregulation of autophagy in isolated guinea pig hearts. J Anesth 28:593–600
Kanamori H, Takemura G, Goto K, Maruyama R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S (2011) The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res 91:330–339
Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE (2009) Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 54:2435–2446
Zhang YJ, Yang SH, Li MH, Iqbal J, Bourantas CV, Mi QY, Yu YH, Li JJ, Zhao SL, Tian NL, Chen SL (2014) Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: role of autophagy. Clin Exp Pharmacol Physiol 41:995–1002
Watanabe T, Takemura G, Kanamori H, Goto K, Tsujimoto A, Okada H, Kawamura I, Ogino A, Takeyama T, Kawaguchi T, Morishita K, Ushikoshi H, Kawasaki M, Mikami A, Fujiwara T, Fujiwara H, Minatoguchi S (2014) Restriction of food intake prevents postinfarction heart failure by enhancing autophagy in the surviving cardiomyocytes. Am J Pathol 184:1384–1394
Kanamori H, Takemura G, Goto K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Morishita K, Kawasaki M, Mikami A, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S (2013) Resveratrol reverses remodeling in hearts with large, old myocardial infarctions through enhanced autophagy-activating AMP kinase pathway. Am J Pathol 182:701–713
Campos JC, Queliconi BB, Bozi LHM, Bechara LRG, Dourado PMM, Andres AM, Jannig PR, Gomes KMS, Zambelli VO, Rocha-Resende C, Guatimosim S, Brum PC, Mochly-Rosen D, Gottlieb RA, Kowaltowski AJ, Ferreira JCB (2017) Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 13:1304–1317
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, PE MB, JJ MM, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:e240–e327
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306:990–995
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N (2004) The role of autophagy during the early neonatal starvation period. Nature 432:1032–1036
Kanamori H, Takemura G, Maruyama R, Goto K, Tsujimoto A, Ogino A, Li L, Kawamura I, Takeyama T, Kawaguchi T, Nagashima K, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S (2009) Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. Am J Pathol 174:1705–1714
Clarke PG (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H (2001) Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J 65:965–968
Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF (2005) Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A 102:13807–13812
Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H (2006) Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol 168:386–397
Hamacher-Brady A, Brady NR, Gottlieb RA (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281:29776–29787
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922
Maruyama R, Goto K, Takemura G, Ono K, Nagao K, Horie T, Tsujimoto A, Kanamori H, Miyata S, Ushikoshi H, Nagashima K, Minatoguchi S, Fujiwara T, Fujiwara H (2008) Morphological and biochemical characterization of basal and starvation-induced autophagy in isolated adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 295:H1599–H1607
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624
Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, Ogino A, Tsujimoto A, Goto K, Maruyama R, Kawasaki M, Mikami A, Fujiwara T, Fujiwara H, Minatoguchi S (2012) Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res 96:456–465
Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M, Seishima M, Minatoguchi S (2015) Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 11:1146–1160
Takemura G, Miyata S, Kawase Y, Okada H, Maruyama R, Fujiwara H (2006) Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy 2:212–214
Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406:902–906
Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M (2000) Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 406:906–910
Higashi K, Yamada Y, Minatoguchi S, Baba S, Iwasa M, Kanamori H, Kawasaki M, Nishigaki K, Takemura G, Kumazaki M, Akao Y, Minatoguchi S (2015) MicroRNA-145 repairs infarcted myocardium by accelerating cardiomyocyte autophagy. Am J Physiol Heart Circ Physiol 309:H1813–HH826
Wei H, Guan JL (2012) Pro-tumorigenic function of autophagy in mammary oncogenesis. Autophagy 8:129–131
Weber KT, Anversa P, Armstrong PW, Brilla CG, Burnett JC Jr, Cruickshank JM, Devereux RB, Giles TD, Korsgaard N, Leier CV, Mendelsohn FAO, Motz WH, Mulvany MJ, Strauer BE (1992) Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol 20:3–16
Zak R (1974) Development and proliferative capacity of cardiac muscle cells. Circ Res 35:17–26
Nag AC (1980) Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28:41–61
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120
Acknowledgments
This study was supported in part by Research Grant from Asahi University and Grant-in-Aid for Scientific Research (16K09509) from the Ministry of Educational, Cultural, Sports, Science and Technology of Japan. The authors thank Yasuaki Hotta, Akiko Niwa, Rieko Hori, and Norie Soga at Asahi University for their technical and secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takemura, G., Kanamori, H., Okada, H. et al. Anti-apoptosis in nonmyocytes and pro-autophagy in cardiomyocytes: two strategies against postinfarction heart failure through regulation of cell death/degeneration. Heart Fail Rev 23, 759–772 (2018). https://doi.org/10.1007/s10741-018-9708-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9708-x