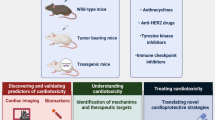
Abstract
Although treatment for heart failure induced by cancer therapy has improved in recent years, the prevalence of cardiomyopathy due to antineoplastic therapy remains significant worldwide. In addition to traditional mediators of myocardial damage, such as reactive oxygen species, new pathways and target cells should be considered responsible for the impairment of cardiac function during anticancer treatment. Accordingly, there is a need to develop novel therapeutic strategies to protect the heart from pharmacologic injury, and improve clinical outcomes in cancer patients. The development of novel protective therapies requires testing putative therapeutic strategies in appropriate animal models of chemotherapy-induced cardiomyopathy. This Position Paper of the Working Group on Drug Cardiotoxicity and Cardioprotection of the Italian Society of Cardiology aims to: (1) define the distinctive etiopatogenetic features of cardiac toxicity induced by cancer therapy in humans, which include new aspects of mitochondrial function and oxidative stress, neuregulin-1 modulation through the ErbB receptor family, angiogenesis inhibition, and cardiac stem cell depletion and/or dysfunction; (2) review the new, more promising therapeutic strategies for cardioprotection, aimed to increase the survival of patients with severe antineoplastic-induced cardiotoxicity; (3) recommend the distinctive pathological features of cardiotoxicity induced by cancer therapy in humans that should be present in animal models used to identify or to test new cardioprotective therapies.
Similar content being viewed by others
Abbreviations
- CHF:
-
Congestive heart failure
- ROS:
-
Reactive oxygen species
- NRG-1:
-
Neuregulin-1
- Top2:
-
Topoisomerase II
- PI3:
-
Phosphoinositide-3
- AKT:
-
Protein kinase B
- MEK/ERK:
-
Mitogen extracellular kinase
- TK:
-
Tyrosine kinase
- VEGFR:
-
Vascular endothelial growth factor receptor
- PDGFR:
-
Platelet-derived growth factor receptor
- CSCs:
-
Cardiac stem cells
- CPCs:
-
Cardiac progenitor cells
- ACE:
-
Angiotensin-converting enzyme
References
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97:2869–2879
Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH (2007) Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 25:3808–3815
Ewer SM, Ewer MS (2008) Cardiotoxicity profile of trastuzumab. Drug Saf 31:459–467
Sawyer DB, Zup**er C, Miller TA, Eppenberger HM, Suter TM (2002) Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 105:1551–1554
Outomuro D, Grana DR, Azzato F, Milei J (2007) Adriamycin-induced myocardial toxicity: new solutions for an old problem? Int J Cardiol 117:6–15
Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE (2005) Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol 131:561–578
Olson RD, Mushlin PS (1990) Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J 4:3076–3086
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229
Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y et al (2007) Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 67:8839–8846
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF et al (2012) Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18:1639–1642
Pointon AV, Walker TM, Phillips KM, Luo J, Riley J, Zhang SD et al (2010) Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS One 5:e12733
Gonzalvez F, Gottlieb E (2007) Cardiolipin: setting the beat of apoptosis. Apoptosis 12:877–885
Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC (2005) Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111:1601–1610
Di X, Gennings C, Bear HD, Graham LJ, Sheth CM, White KL Jr et al (2010) Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat 124:349–360
Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P (2007) Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res 75:168–177
Pentassuglia L, Sawyer DB (2009) The role of neuregulin-1 beta/erbb signaling in the heart. Exp Cell Res 315:627–637
Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5:341–354
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Suter TM, Ewer MS (2013) Cancer drugs and the heart: importance and management. Eur Heart J 34:1102–1111
Ewer MS, Ewer SM (2010) Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction. J Clin Oncol 28:3901–3904
Odiete O, Hill MF, Sawyer DB (2012) Neuregulin in cardiovascular development and disease. Circ Res 111:1376–1385
Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ (2013) Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res 113:754–764
Bersell K, Arab S, Haring B, Kuhn B (2009) Neuregulin-1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138:257–270
Force T, Krause DS, Van Etten RA (2007) Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 7:332–344
De Keulenaer GW, Doggen K, Lemmens K (2010) The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res 106:35–46
Tocchetti CG, Ragone G, Coppola C, Rea D, Piscopo G, Scala S et al (2012) Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. Eur J Heart Fail 14:130–137
Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD et al (2012) Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 13:1–10
Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y et al (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8:459–465
Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V et al (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 23:7820–7826
Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM et al (2006) Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol 41:845–854
Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C et al (2008) Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 26:5204–5212
Welti J, Loges S, Dimmeler S, Carmeliet P (2013) Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 123:3190–3200
Cheng H, Force T (2010) Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res 106:21–34
Tocchetti CG, Gallucci G, Coppola C, Piscopo G, Cipresso C, Maurea C et al (2013) The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. Eur J Heart Fail 15:482–489
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT et al (2012) Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol 23(Suppl 7):vii155–vii166
Marone G, Granata F (2014) Angiogenesis, lymphangiogenesis and clinical implications. Preface. Chem Immunol Allergy 99:XI–XII
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Gresset S, Westermeier P, Rademacher S, Ouzunova M, Presterl T, Westhoff P et al (2010) Stable carbon isotope discrimination is under genetic control in the C4 species maize with several genomic regions influencing trait expression. Plant Physiol 164:131–143
Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL et al (2010) Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest 120:472–484
Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L et al (2007) Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370:2011–2019
Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C et al (2008) Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer 112:2500–2508
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Telli ML, Witteles RM, Fisher GA, Srinivas S (2008) Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 19:1613–1618
Hasinoff BB, Patel D (2010) The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol Appl Pharmacol 249:132–139
Anisimov A, Alitalo A, Korpisalo P, Soronen J, Kaijalainen S, Leppanen VM et al (2009) Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ Res 104:1302–1312
Loges S, Roncal C, Carmeliet P (2009) Development of targeted angiogenic medicine. J Thromb Haemost 7:21–33
Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K (2006) Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension 47:887–893
Levy BI (2006) Microvascular plasticity and experimental heart failure. Hypertension 47:827–829
De Boer RA, Pinto YM, Van Veldhuisen DJ (2003) The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation 10:113–126
Kerkela R, Woulfe KC, Durand JB, Vagnozzi R, Kramer D, Chu TF et al (2009) Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci 2:15–25
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102
Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A et al (2007) Human cardiac stem cells. Proc Natl Acad Sci USA 104:14068–14073
De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L et al (2010) Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121:276–292
Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P et al (2006) Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 116:1865–1877
Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM (2004) Antioxidants and cancer therapy: a systematic review. J Clin Oncol 22:517–528
van Dalen EC, Caron HN, Dickinson HO, Kremer LC (2008) Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev (2):CD003917. doi:10.1002/14651858.CD003917.pub3
Huelsenbeck J, Henninger C, Schad A, Lackner KJ, Kaina B, Fritz G (2011) Inhibition of Rac1 signaling by lovastatin protects against anthracycline-induced cardiac toxicity. Cell Death Dis 2:e190
Riad A, Bien S, Westermann D, Becher PM, Loya K, Landmesser U et al (2009) Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res 69:695–699
Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH (2012) Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol 60:2384–2390
Acar Z, Kale A, Turgut M, Demircan S, Durna K, Demir S et al (2011) Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol 58:988–989
Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW (2011) Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation 124:642–650
Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR (2009) Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 10:598–605
Weijl NI, Elsendoorn TJ, Lentjes EG, Hopman GD, Wipkink-Bakker A, Zwinderman AH et al (2004) Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. Eur J Cancer 40:1713–1723
Giovannucci E, Chan AT (2010) Role of vitamin and mineral supplementation and aspirin use in cancer survivors. J Clin Oncol 28:4081–4085
Hardy ML (2008) Dietary supplement use in cancer care: help or harm. Hematol Oncol Clin N Am 22:581–617
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G et al (2010) Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 55:213–220
Okumura K, ** D, Takai S, Miyazaki M (2002) Beneficial effects of angiotensin-converting enzyme inhibition in adriamycin-induced cardiomyopathy in hamsters. Jpn J Pharmacol 88:183–188
Tokudome T, Mizushige K, Noma T, Manabe K, Murakami K, Tsuji T et al (2000) Prevention of doxorubicin (adriamycin)-induced cardiomyopathy by simultaneous administration of angiotensin-converting enzyme inhibitor assessed by acoustic densitometry. J Cardiovasc Pharmacol 36:361–368
Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H et al (2004) Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The task force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 25:1454–1470
Cernecka H, Ochodnicka-Mackovicova K, Kucerova D, Kmecova J, Nemcekova V, Doka G et al (2013) Enalaprilat increases PPARbeta/delta expression, without influence on PPARalpha and PPARgamma, and modulate cardiac function in sub-acute model of daunorubicin-induced cardiomyopathy. Eur J Pharmacol 714:472–477
Soga M, Kamal FA, Watanabe K, Ma M, Palaniyandi S, Prakash P et al (2006) Effects of angiotensin II receptor blocker (candesartan) in daunorubicin-induced cardiomyopathic rats. Int J Cardiol 110:378–385
Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K et al (2005) Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 104:2492–2498
Shi Y, Moon M, Dawood S, McManus B, Liu PP (2011) Mechanisms and management of doxorubicin cardiotoxicity. Herz 36:296–305
Bovelli D, Plataniotis G, Roila F (2010) Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann Oncol 21(Suppl 5):v277–v282
Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH (2013) Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ Heart Fail 6:420–426
Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M et al (2013) Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol 167:2306–2310
Oliva S, Cioffi G, Frattini S, Simoncini EL, Faggiano P, Boccardi L et al (2012) Administration of angiotensin-converting enzyme inhibitors and beta-blockers during adjuvant trastuzumab chemotherapy for nonmetastatic breast cancer: marker of risk or cardioprotection in the real world? Oncologist 17:917–924
Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A et al (2006) Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 48:2258–2262
Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM et al (2013) Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 61:2355–2362
Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N, Marinakis T et al (2012) Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Cardiology 123:240–247
Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD et al (2008) Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA 105:14555–14560
Zhang X, Szeto C, Gao E, Tang M, ** J, Fu Q et al (2013) Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 112:498–509
Nakamura K, Kusano K, Nakamura Y, Kakishita M, Ohta K, Nagase S et al (2002) Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation 105:2867–2871
Heck SL, Gulati G, Ree AH, Schulz-Menger J, Gravdehaug B, Rosjo H et al (2012) Rationale and design of the prevention of cardiac dysfunction during an adjuvant breast cancer therapy (PRADA) trial. Cardiology 123:240–247
Pituskin E, Haykowsky M, Mackey JR, Thompson RB, Ezekowitz J, Koshman S et al (2011) Rationale and design of the multidisciplinary approach to novel therapies in cardiology oncology research trial (MANTICORE 101-breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer 11:318
Oliveira MS, Melo MB, Carvalho JL, Melo IM, Lavor MS, Gomes DA et al (2013) Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. J Cancer Sci Ther 5:52–57
Di GH, Jiang S, Li FQ, Sun JZ, Wu CT, Hu X et al (2012) Human umbilical cord mesenchymal stromal cells mitigate chemotherapy-associated tissue injury in a pre-clinical mouse model. Cytotherapy 14:412–422
Merino H, Singla DK (2014) Notch-1 mediated cardiac protection following embryonic and induced pluripotent stem cell transplantation in doxorubicin-induced heart failure. PLoS One 9:e101024
Singla DK (2014) Akt-mTOR pathway inhibits apoptosis and fibrosis in doxorubicin-induced cardiotoxicity following embryonic stem cell transplantation. Cell Transplant 24(6):1031–1042
Singla DK, Abdelli LS (2014) Embryonic stem cells and released factors stimulate c-kit/FLK-1 progenitor cells and promote neovascularization in doxorubicin-induced cardiomyopathy. Cell Transplant 24(6):1043–1052
Madonna R, Rokosh G, De Caterina R, Bolli R (2010) Hepatocyte growth factor/Met gene transfer in cardiac stem cells—potential for cardiac repair. Basic Res Cardiol 105:443–452
Herman EH, Ferrans VJ (1998) Preclinical animal models of cardiac protection from anthracycline-induced cardiotoxicity. Semin Oncol 25:15–21
Lipshultz SE, Cohen H, Colan SD, Herman EH (2006) The relevance of information generated by in vitro experimental models to clinical doxorubicin cardiotoxicity. Leuk Lymphoma 47:1454–1458
Zbinden G, Bachmann E, Holderegger C (1971) Model systems for cardiotoxic effects of anthracyclines. Antibiot Chemother 1978(23):255–270
Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M et al (2006) Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114:2474–2481
Piegari E, Di Salvo G, Castaldi B, Vitelli MR, Rodolico G, Golino P et al (2008) Myocardial strain analysis in a doxorubicin-induced cardiomyopathy model. Ultrasound Med Biol 34:370–378
Adamcova M, Simunek T, Kaiserova H, Popelova O, Sterba M, Potacova A et al (2007) In vitro and in vivo examination of cardiac troponins as biochemical markers of drug-induced cardiotoxicity. Toxicology 237:218–228
Madonna R, Delli Pizzi S, Di Donato L, Mariotti A, Di Carlo L, D’Ugo E et al (2012) Non-invasive in vivo detection of peripheral limb ischemia improvement in the rat after adipose tissue-derived stromal cell transplantation. Circ J 76:1517–1525
Madonna R, Delli Pizzi S, Tartaro A, De Caterina R (2014) Transplantation of mesenchymal cells improves peripheral limb ischemia in diabetic rats. Mol Biotechnol 56:438–448
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Madonna, R., Cadeddu, C., Deidda, M. et al. Improving the preclinical models for the study of chemotherapy-induced cardiotoxicity: a Position Paper of the Italian Working Group on Drug Cardiotoxicity and Cardioprotection. Heart Fail Rev 20, 621–631 (2015). https://doi.org/10.1007/s10741-015-9497-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9497-4