Abstract
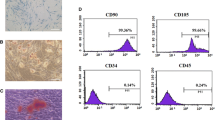
Bone regeneration is impaired in patients with type 2 diabetes mellitus (T2DM), which leads to non-healing after bone loss. The decreased osteogenic capacity of bone mesenchymal stem cells (BMSCs) might be a main reason. Sema3A, as a powerful protein promoting osteocyte differentiation, shows potential for bone regeneration treatment. BMSCs may be a therapeutic solution. In this study, we divided BMSCs from T2DM rats (BMSCs-D) and normal rats (BMSCs-N), identified their ability to differentiate into different cell types. Then we found decreased expression of Sema3A in BMSCs-D compared with BMSCs-N. Stimulating with Sema3A showed no influence in the proliferation or migration of BMSCs. However, Sema3A stimulation significantly increased the expression of osteogenic‑related genes, including type I collagen, alkaline phosphatase, Runt-related transcription factor 2 (RUNX2), bone morphogenetic protein and osteocalcin. Besides, the osteogenic capacity of BMSCs was also increased by Sema3A stimulation. In conclusion, we proved that exogenous Sema3A stimulation might repair the osteogenic capacity of BMSCs-D, thus providing a new strategy for restoring the impaired bone regeneration ability for T2DM patients.




Similar content being viewed by others
References
Abraham NG, Li M, Vanella L, Peterson SJ, Ikehara S, Asprinio D (2008) Bone marrow stem cell transplant into intra-bone cavity prevents type 2 diabetes: role of heme oxygenase-adiponectin. J Autoimmun 30:128–135
Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC (1996) Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383:525–528
Bhaskar B, Mekala NK, Baadhe RR, Rao PS (2014) Role of signaling pathways in mesenchymal stem cell differentiation. Curr Stem Cell Res Ther 9:508–512
Camargo WA, de Vries R, van Luijk J, Hoekstra JW, Bronkhorst EM, Jansen JA, van den Beucken J (2017). Diabetes mellitus and bone regeneration: A systematic review and meta-analysis of animal studies. Tissue Eng Part B Rev 23, 471–479
Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R (2010) Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 19:1875–1884
Dacquin R, Starbuck M, Schinke T, Karsenty G (2002) Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn 224:245–251
Deng Y, Guo T, Li J, Guo L, Gu P, Fan X (2017). Repair of calvarial bone defect using jarid1a-knockdown bone mesenchymal stem cells in rats. Tissue Eng Part A. https://doi.org/10.1089/ten.tea.2017.0168
Fang K, Song W, Wang L, Jia S, Wei H, Ren S, Xu X, Song Y (2014) Immobilization of chitosan film containing semaphorin 3A onto a microarc oxidized titanium implant surface via silane reaction to improve MG63 osteogenic differentiation. Int J Nanomed 9:4649–4657
Fang K, Song W, Wang L, Xu X, Tan N, Zhang S, Wei H, Song Y (2016) Semaphorin 3A-modified adipose-derived stem cell sheet may improve osseointegration in a type 2 diabetes mellitus rat model. Mol Med Rep 14:2449–2456
Fiorellini JP, Chen PK, Nevins M, Nevins ML (2000) A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent 20:366–373
Gopalakrishnan V, Vignesh RC, Arunakaran J, Aruldhas MM, Srinivasan N (2006) Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol 84:93–101
Gusić N, Ivković A, VaFaye J, Vukasović A, Ivković J, Hudetz D, Janković S (2014) Nanobiotechnology and bone regeneration: a mini-review. Int Orthop 38:1877–1884
Hamann C, Goettsch C, Mettelsiefen J, Henkenjohann V, Rauner M, Hempel U, Bernhardt R, Fratzl-Zelman N, Roschger P, Rammelt S et al (2011) Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am J Physiol Endocrinol Metab 301:E1220-E1228
Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H (2012). Osteoprotection by semaphorin 3A. Nature 485:69–74
He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT (2004). Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 145:447–452
Kawashima Y, Fritton JC, Yakar S, Epstein S, Schaffler MB, Jepsen KJ, LeRoith D (2009). Type 2 diabetic mice demonstrate slender long bones with increased fragility secondary to increased osteoclastogenesis. Bone 44:648–655
Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, Goodman CS (1992) Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 9:831–845
Lapmanee S, Charoenphandhu N, Aeimlapa R, Suntornsaratoon P, Wongdee K, Tiyasatkulkovit W, Kengkoom K, Chaimongkolnukul K, Seriwatanachai D, Krishnamra N (2014) High dietary cholesterol masks type 2 diabetes-induced osteopenia and changes in bone microstructure in rats. Lipids 49:975–986
Li Z, Hao J, Duan X, Wu N, Zhou Z, Yang F, Li J, Zhao Z, Huang S (2017) The role of semaphorin 3A in bone remodeling. Front Cell Neurosci 11:40
Lin J, Shao J, Juan L, Yu W, Song X, Liu P, Weng W, Xu J, Mehl C (2017). Enhancing bone regeneration by combining mesenchymal stem cell sheets with beta-TCP/COL-I scaffolds. J Biomed Mater Res B Appl Biomater. https://doi.org/10.1002/jbm.b.34003
Liu X, Tan N, Zhou Y, Zhou X, Chen H, Wei H, Chen J, Xu X, Zhang S, Yang G et al. (2016). Semaphorin 3A shifts adipose mesenchymal stem cells towards osteogenic phenotype and promotes bone regeneration in vivo. Stem Cells Int 2016:2545214
Liu Y, Yang X, Maureira P, Falanga A, Marie V, Gauchotte G, Poussier S, Groubatch F, Marie PY, Tran N (2017) Permanently hypoxic cell culture yields rat bone marrow mesenchymal cells with higher therapeutic potential in the treatment of chronic myocardial infarction. Cell Physiol Biochem 44:1064–1077
Luo Y, Raible D, Raper JA (1993) Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75:217–227
Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper JA (1995) A family of molecules related to collapsin in the embryonic chick nervous system. Neuron 14:1131–1140
Marin C, Luyten FP, Van der Schueren B, Kerckhofs G, Vandamme K (2018). The impact of type 2 diabetes on bone fracture healing. Front Endocrinol (Lausanne) 9:6
Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P (2010) Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31:3572–3579
Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castano-Betancourt MC, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA et al (2013) High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabet Care 36:1619–1628
Rodda SJ, McMahon AP (2006). Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244
Salah RA, Mohamed IK, El-Badri N (2018). Development of decellularized amniotic membrane as a bioscaffold for bone marrow-derived mesenchymal stem cells: ultrastructural study. J Mol Histol 49(3):289–301
Sun XK, Zhou J, Zhang L, Ma T, Wang YH, Yang YM, Tang YT, Li H, Wang LJ (2017) Down-regulation of Noggin and miR-138 coordinately promote osteogenesis of mesenchymal stem cells. J Mol Histol 48:427–436
Tang P, Yin P, Lv H, Zhang L, Zhang L (2015) The Role of Semaphorin 3A In The Skeletal System. Crit Rev Eukaryot Gene Expr 25:47–57
Villarino ME, Sanchez LM, Bozal CB, Ubios AM (2006) Influence of short-term diabetes on osteocytic lacunae of alveolar bone. A histomorphometric study. Acta Odontol Latinoam 19:23–28
Yan W, Li X (2013) Impact of diabetes and its treatments on skeletal diseases. Front Med 7:81–90
Ye Y, Feng TT, Peng YR, Hu SQ, Xu T (2018) The treatment of spinal cord injury in rats using bone marrow-derived neural-like cells induced by cerebrospinal fluid. Neurosci Lett 666:85–91
Zhang HL, **e XF, **ong YQ, Liu SM, Hu GZ, Cao WF, Wu XM (2018) Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res 1680:143–154
Zhou W, Liu Q, Xu B (2018) Improvement of bone defect healing in rats via mesenchymal stem cell supernatant. Exp Ther Med 15:1500–1504
Acknowledgements
This study is supported by the National Natural Science Foundation of China [NO. 81470775]. Department of Burns and Cutaneous Surgery provided most of the equipment of experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Qiao, Q., Xu, X., Song, Y. et al. Semaphorin 3A promotes osteogenic differentiation of BMSC from type 2 diabetes mellitus rats. J Mol Hist 49, 369–376 (2018). https://doi.org/10.1007/s10735-018-9776-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9776-1