Abstract
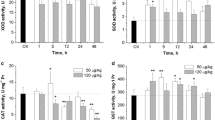
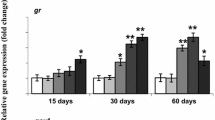
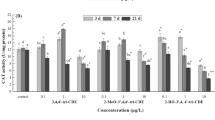
The worldwide occurrence of cyanobacterial blooms makes it necessary to perform environmental risk assessment procedures to monitor the effects of microcystins (MCs) on fish. Oxidative stress biomarkers are valuable tools in this regard. In the present study, phytoplanktivorous bighead carp (Aristichthys nobilis) were injected intraperitoneally (i.p.) with extracted MCs (mainly MC-RR and -LR) at two doses, 400 and 1,000 μg kg−1 bw, and antioxidant responses of the liver as biomarkers of oxygen-mediated toxicity were studied at 1, 3, 12, 24 and 48 h after injection. Contents of reactive oxygen species (ROS) and activities of antioxidant enzymes [catalase (CAT), superoxide dismutase (SOD), glutathione peroxide (GPX), and glutathione reductase (GR)] as well as glutathione S-transferase (GST) in the liver in both dose groups showed a biphasic change with an increase at initial 3 h followed by a decrease after injection, owing to the roles of the antioxidant system in eliminating excessive ROS and regenerating glutathione (GSH). The increased GST was probably due to the high transcription of cytosolic GST α and ρ, suggesting the importance of MCs detoxification by GSH pathway. The stable GSH levels in liver may be explained by the high basic GSH concentration in liver, and/or an increased GSH synthesis, suggesting a high ability to detoxify MCs and to release associated high oxidative pressure in phytoplantivorous fish.


Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, vol 105. Academic Press, Orlando, pp 121–126
Bayer WF, Fridovich JL (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Best JH, Pflugmacher S, Wiegand C, Eddy FB, Metcalf JS, Codd GA (2002) Effects of enteric bacterial and cyanobacterial lipopolysaccharides, and of microcystin-LR, on glutathione S-transferase activities in zebrafish (Danio rerio). Aquat Toxicol 60:223–231
Blaha L, Kopp R, Simkova K, Mares J (2004) Oxidative stress biomarkers are modulated in silver carp (Hypophthalmichthys molitrix Val) exposed to microcystin-producing cyanobacterial water bloom. Acta Vet Brno 73:477–482
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye-binding. Anal Biochem 72:248–254
Cazenave J, Bistoni MA, Pesce SF, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus exposed to Microcystin-RR. Aquat Toxicol 76:1–12
Chen J, **e P, Zhang DW, Lei HH (2007) In situ studies on the distribution patterns and dynamics of microcystins in biomanipulation fish-bighead carp (Aristichthys nobilis). Environ Pollut 147:150–157
Codd GA, Ward CJ, Bell SG (1997) Cyanobacterial toxins: occurrence, modes of action health effects and exposure routes. In: Seiler JP, Vilanova E (eds) Applied toxicology. Springer, Berlin, pp 399–410
De Figereido DR, Azeiteiro UM, Esteves SM, Goncalves FJM, Pereira JM (2004) Microcystin-producing blooms—a serious global public health issue. Ecotoxicol Environ Saf 59:151–163
Ding W-X, Shen H-M, Zhu HG, Ong C-N (1998) Studies on oxidative damage induced by cyanobacteria extract in primary cultured rat hepatocytes. Environ Res 78:12–18
Ding W-X, Shen H-M, Ong C-N (2000) Critical role of reactive oxygen species and mitochondrial permeability transition in microcystin-induced rapid apoptosis in rat hepatocytes. J Toxicol Environ Health A 64:507–519
Ernst B, Hitzfeld BC, Dietrich DR (2001) Presence of Planktothrix sp. and cyanobacterial toxins in Lake Ammersee, Germany and their impact on whitefish (Coregonus lavaretus L.). Environ Toxicol 16:483–488
Fu J, **e P (2006) The acute effects of microcystin LR on the transcription of nine glutathione S-transferase genes in common carp Cyprinus carpio L. Aquat Toxicol 80(3):261–266
Gehringer MM, Shephard EG, Downing TG, Wiegand C, Neilan BA (2004) An investigation into the detoxification of microcystin-LR by the glutathione pathway in Balb/c mice. Int J Biochem Cell Biol 36:931–941
Hooser SB (2000) Fulminant hepatocyte apoptosis in vivo following microcystin-LR administration to rats. Toxicol Pathol 28:726–733
Jos Á, Pichardo S, Prieto AI, Repetto G, Vázquez CM, Moreno I, Cameán AM (2005) Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat Toxicol 72:261–271
Li XY, Liu YD, Song LR, Liu J (2003) Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon 42:85–89
Li XY, Chung I, Kim J, Lee J (2005) Oral exposure to Microcystis increases activity-augmented antioxidant enzymes in the liver of loach (Misgurnus mizolepis) and has no effects on lipid peroxidation. Comp Biochem Physiol C 141:292–296
Li L, **e P, Chen J (2005) In vivo studies on toxin accumulation in liver and ultrastructural changes of hepatocytes of the phytoplantivorous bighead carp i.p.-injected with extracted microcystins. Toxicon 46:533–546
Malbrouck C, Trausch G, Devos P, Kestemont P (2004) Effect of microcystin-LR on protein phosphatase activity in fed and fasted juvenile goldfish Carassius auratus L. Toxicon 43:295–301
Moreno I, Pichardo S, Jos Á, Gomez-Amores L, Mate A, Vázquez CM, Cameán AM (2005) Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 45:395–402
Park HD, Kim B, Kim E, Okino T (1998) Hepatotoxic microcystins and neurotoxic anatoxin-a in cyanobacterial blooms from Korean lakes. Environ Toxicol Water Qual 13:225–234
Pflugmacher S, Wiegand C, Oberemm A, Beattie KA, Krause E, Codd GA, Steinberg CEW (1998) Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: the first step of detoxication. Biochim Biophys Acta 1425:527–533
Pinho GLL, Rosa MC, Maciel FE, Bianchini A, Yunes JS, Proenca LAO, Monserrat JM (2005) Antioxidant responses and oxidative stress after microcystin exposure in the hepatopancreas of an estuarine crab species. Ecotoxicol Environ Saf 61:353–360
Pireo AI, Jos Á, Pichardo S, Moreno IM, Cameán AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–321
Prieto AI, Pichardo S, Jos Á, Moreno I, Cameán AM (2007) Time-dependent oxidative stress response after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions. Aquat Toxicol 84:337–345
Snyder GS, Goodwin AE, Freeman DW (2002) Evidence that channel catfish, Ictalurus punctatus (Rafinesque) mortality is not linked to ingestion of the hepatotoxin MC-LR. J Fish Dis 25:275–285
Takenaka S, Otsu R (1999) Effects of L-cysteine and reduced glutathione on the toxicities of microcystin-LR: the effect for acute liver failure and inhibition of protein phosphatase activity. Aquat Toxicol 48:65–68
Wiegand C, Pflugmacher S (2005) Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol Appl Pharmacol 203:201–218
Wiegand C, Pflugmacher S, Oberemm A, Meems N, Beattie KA, Steinberg C, Cood GA (1999) Uptake and effects of microcystin-LR on detoxification enzymes during the ontogenesis of the zebrafish (Danio rerio). Int Rev Hydrobiol 85:413–422
Wilhelm Filho D (1996) Fish antioxidant defenses. A comparative approach. Braz J Med Biol Res 29:1735–1742
**e P, Liu JK (2001) Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: synthesis of decades of research and application in a subtropical hypereutrophic lake. J Sci World 1:337–356
**e LQ, **e P, Ozawa K, Honma T, Yokoyama A, Park HD (2004) Dynamics of microcystins-LR and -RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Environ Pollut 127:431–439
Xu LH, Chen GS, Chen JP, Xu JM, Zhang YY (1998) Toxic effects of microcystin on fish liver. Acta Hydrobiol Sin 22:378–379 (in Chinese)
Acknowledgements
The authors would like to thank Dr H.J.T. Goos and the anonymous reviewers for their useful comments and suggestions on the manuscript. This work was supported by the Key Project of CAS titled “The effects of the regenerative organic pollutant microcystins on the safety of aquatic food” and by a fund from the National Natural Science Foundation of China (30225011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., **e, P. & Guo, L. Antioxidant response in liver of the phytoplanktivorous bighead carp (Aristichthys nobilis) intraperitoneally-injected with extracted microcystins. Fish Physiol Biochem 36, 165–172 (2010). https://doi.org/10.1007/s10695-008-9228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9228-z