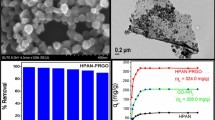
Abstract
The results of investigations about the polymerization of p-aminophenol in neutral-weak alkaline medium (pH = 7–8) in our lab showed that the produced polymer which was insoluble in water and soluble in methanol has high tendency to form selectively a blue complex with Ni(II). Investigations into the chemical structure of polymer showed that polymer has a special structure, similar to polyamine in which the aromatic rings are connected through O-bridges. Based on these data, it was decided to polymerize p-aminophenol in situ on graphene oxide (GO) and use as a new sorbent for selective separation and preconcentration of trace amount of Ni(II) from water samples. By this, the rate of sorption of Ni(II) will also be increased considerably with respect to GO alone. Resulting composite (GO-Pp-AP) was characterized by FT-IR, XRD, FE-SEM, and EDS. The obtained data confirmed the uniform growth of the polymer on the GO and the absence of granular particles. The composite shows high tendency and high rate of sorption of Ni(II) and consequently was utilized for solid phase extraction (SPE) of Ni(II) ions before its determination by flame atomic absorption (FAAS). The effects of important parameters on the recovery of Ni(II) were investigated. The presence of foreign ions has no meaningful effect on the recovery percentage of Ni(II). Under the optimum conditions, limit of detection and relative standard deviation were found to be 0.70 μg L−1 and 1.8% (for n = 6; at 20 μg L−1 of Ni(II)), respectively. Testing the standard reference material and analyzing the spiked real samples exhibit that the procedure can be successfully employed for determination of Ni(II) in natural water and wastewater samples.

Graphical abstract





Similar content being viewed by others
References
Baki, M. H., & Shemirani, F. (2013). Surfactant modified walnut sawdust as an alternative green support for efficient preconcentration of nickel ions from different real samples. Analytical Methods, 5(13), 3255–3263.
Baytak, S., & Türker, A. R. (2006). Determination of lead and nickel in environmental samples by flame atomic absorption spectrometry after column solid-phase extraction on Ambersorb-572 with EDTA. Journal of Hazardous Materials, 129(1–3), 130–136.
Camel, V. (2003). Solid phase extraction of trace elements. Spectrochimica Acta Part B: Atomic Spectroscopy, 58(7), 1177–1233.
Carter, S., Fisher, A. S., Goodall, P. S., Hinds, M. W., Lancaster, S., & Shore, S. (2009). Atomic spectrometry update. Industrial analysis: metals, chemicals and advanced materials. Journal of Analytical Atomic Spectrometry, 24(12), 1599–1656.
Chandra, V., & Kim, K. S. (2011). Highly selective adsorption of Hg 2+ by a polypyrrole–reduced graphene oxide composite. Chemical Communications, 47(13), 3942–3944.
Chiou, N.-R., Lu, C., Guan, J., Lee, L. J., & Epstein, A. J. (2007). Growth and alignment of polyaniline nanofibres with superhydrophobic, superhydrophilic and other properties. Nature Nanotechnology, 2(6), 354–357.
Dimiev, A. M., & Tour, J. M. (2014). Mechanism of graphene oxide formation. ACS Nano, 8(3), 3060–3068.
Dizavandi, Z. R., Aliakbar, A., & Sheykhan, M. (2017). Electrocatalytic determination of clopidogrel using Bi2O3-Pp-AP/GCE by differential pulse voltammetry in pharmaceutical productions. Journal of Electroanalytical Chemistry, 805, 24–31.
Duda-Chodak, A., & Blaszczyk, U. (2008). The impact of nickel on human health. Journal of Elementology, 13(4), 685–693.
Fabec, J. L., & Ruschak, M. L. (1985). Determination of nickel, vanadium, and sulfur in crudes and heavy crude fractions by inductively coupled argon plasma atomic emission spectrometry and flame atomic absorption spectrometry. Analytical Chemistry, 57(9), 1853–1863.
Ferreira, S. L., de Andrade, J. B., Maria das Graças, A. K., Pereira, M. d. G., Lemos, V. A., dos Santos, W. N., et al. (2007). Review of procedures involving separation and preconcentration for the determination of cadmium using spectrometric techniques. Journal of Hazardous Materials, 145(3), 358–367.
Gonzalez, P., Cortınez, V., & Fontan, C. (2002). Determination of nickel by anodic adsorptive strip** voltammetry with a cation exchanger-modified carbon paste electrode. Talanta, 58(4), 679–690.
Harris, D. C. (2010). Quantitative chemical analysis. New York: W. H. Freeman and Company.
Henriques, B., Gonçalves, G., Emami, N., Pereira, E., Vila, M., & Marques, P. A. (2016). Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. Journal of Hazardous Materials, 301, 453–461.
Jalali, M., & Aliakbar, A. (2013). Electrochemical synthesis and characterization of a new selective chelating agent for Ni (II) and its environmental analytical application. Analytical Methods, 5(22), 6352–6359.
Kojidi, M. H., & Aliakbar, A. (2017). A graphene oxide based poly (2, 6-diaminopyridine) composite for solid-phase extraction of Cd (II) prior to its determination by FAAS. Microchimica Acta, 184(8), 2855–2860.
Kumar, N. A., Choi, H.-J., Shin, Y. R., Chang, D. W., Dai, L., & Baek, J.-B. (2012). Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano, 6(2), 1715–1723.
Latorre, C. H., Méndez, J. Á., García, J. B., Martín, S. G., & Crecente, R. P. (2012). Carbon nanotubes as solid-phase extraction sorbents prior to atomic spectrometric determination of metal species: A review. Analytica Chimica Acta, 749, 16–35.
Liu, Z., Liu, Q., Dai, X., Shen-Tu, C., Yao, C., & Kong, Y. (2013). Synthesis of poly (2, 6-diaminopyridine) using Interface polymerization and the electrochemical properties of poly (2, 6-diaminopyridine). ECS Electrochemistry Letters, 2(7), G1–G4.
Marcano, D. C., Kosynkin, D. V., Berlin, J. M., Sinitskii, A., Sun, Z., Slesarev, A., Alemany, L. B., Lu, W., & Tour, J. M. (2010). Improved synthesis of graphene oxide. ACS Nano, 4(8), 4806–4814.
Martín-Cameán, A., Jos, A., Calleja, A., Gil, F., Iglesias-Linares, A., Solano, E., & Cameán, A. M. (2014). Development and validation of an inductively coupled plasma mass spectrometry (ICP-MS) method for the determination of cobalt, chromium, copper and nickel in oral mucosa cells. Microchemical Journal, 114, 73–79.
Ngeontae, W., Aeungmaitrepirom, W., & Tuntulani, T. (2007). Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb (II), Cu (II), Ni (II), Co (II) and Cd (II). Talanta, 71(3), 1075–1082.
Otero-Romaní, J., Moreda-Piñeiro, A., Bermejo-Barrera, P., & Martin-Esteban, A. (2009). Ionic imprinted polymer for nickel recognition by using the bi-functionalized 5-vinyl-8-hydroxyquinoline as a monomer: Application as a new solid phase extraction support. Microchemical Journal, 93(2), 225–231.
Pourmand, N., Sanagi, M. M., Naim, A. A., Ibrahim, W. A. W., & Baig, U. (2015). Dispersive micro-solid phase extraction method using newly prepared poly (methyl methacrylate) grafted agarose combined with ICP-MS for the simultaneous determination of Cd, Ni, Cu and Zn in vegetable and natural water samples. Analytical Methods, 7(7), 3215–3223.
Ren, X., Wu, Q., Xu, H., Shao, D., Tan, X., Shi, W., Chen, C., Li, J., Chai, Z., Hayat, T., & Wang, X. (2016). New insight into GO, cadmium (II), phosphate interaction and its role in GO colloidal behavior. Environmental Science & Technology, 50(17), 9361–9369.
Schaumlöffel, D. (2012). Nickel species: Analysis and toxic effects. Journal of Trace Elements in Medicine and Biology, 26(1), 1–6.
Shirkhanloo, H., Khaligh, A., Mousavi, H. Z., & Rashidi, A. (2015). Graphene oxide-packed micro-column solid-phase extraction combined with flame atomic absorption spectrometry for determination of lead (II) and nickel (II) in water samples. International Journal of Environmental Analytical Chemistry, 95(1), 16–32.
Sitko, R., Zawisza, B., & Malicka, E. (2013). Graphene as a new sorbent in analytical chemistry. TrAC Trends in Analytical Chemistry, 51, 33–43.
Sitko, R., Janik, P., Feist, B., Talik, E., & Gagor, A. (2014). Suspended aminosilanized graphene oxide nanosheets for selective preconcentration of lead ions and ultrasensitive determination by electrothermal atomic absorption spectrometry. ACS Applied Materials & Interfaces, 6(22), 20144–20153.
Sitko, R., Janik, P., Zawisza, B., Talik, E., Margui, E., & Queralt, I. (2015). Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Analytical Chemistry, 87(6), 3535–3542.
Skoog, D. A., Holler, F. J., & Nieman, T. A. (1998). Principles of instrumental analysis. Saunders College Publishing.
Sunderman, F. W., Jr., Dingle, B., Hopfer, S. M., & Swift, T. (1988). Acute nickel toxicity in electroplating workers who accidently ingested a solution of nickel sulfate and nickel chloride. American Journal of Industrial Medicine, 14(3), 257–266.
Taj, S., Ahmed, M., & Sankarapapavinasam, S. (1992). Poly (para-aminophenol): a new soluble, electroactive conducting polymer. Journal of Electroanalytical Chemistry, 338(1–2), 347–352.
Tuzen, M., & Soylak, M. (2009). Column solid-phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations. Journal of Hazardous Materials, 164(2–3), 1428–1432.
**e, F., Lin, X., Wu, X., & **e, Z. (2008). Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta, 74(4), 836–843.
Xu, J., Wang, K., Zu, S.-Z., Han, B.-H., & Wei, Z. (2010). Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano, 4(9), 5019–5026.
Yang, S.-T., Chang, Y., Wang, H., Liu, G., Chen, S., Wang, Y., Liu, Y., & Cao, A. (2010). Folding/aggregation of graphene oxide and its application in Cu2+ removal. Journal of Colloid and Interface Science, 351(1), 122–127.
Yilmaz, E., & Soylak, M. (2014). Solid phase extraction of Cd, Pb, Ni, Cu, and Zn in environmental samples on multiwalled carbon nanotubes. Environmental Monitoring and Assessment, 186(9), 5461–5468.
Yuan, Y., Zhang, G., Li, Y., Zhang, G., Zhang, F., & Fan, X. (2013). Poly (amidoamine) modified graphene oxide as an efficient adsorbent for heavy metal ions. Polymer Chemistry, 4(6), 2164–2167.
Zawisza, B., Baranik, A., Malicka, E., Talik, E., & Sitko, R. (2016). Preconcentration of Fe (III), Co (II), Ni (II), Cu (II), Zn (II) and Pb (II) with ethylenediamine-modified graphene oxide. Microchimica Acta, 183(1), 231–240.
Zhang, K., Zhang, L. L., Zhao, X., & Wu, J. (2010). Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chemistry of Materials, 22(4), 1392–1401.
Zhao, G., Li, J., Ren, X., Chen, C., & Wang, X. (2011). Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environmental Science & Technology, 45(24), 10454–10462.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• GO-Pp-AP composite was prepared by in situ polymerization of p-aminophenol on graphene oxide as a solid support
• FE-SEM and nitrogen map** analysis confirmed that the GO sheets are finely coated by poly(p-aminophenol)
• It was utilized as a sorbent for SPE of Ni(II) before FAAS determination
• It shows selectivity for Ni(II), rapid mass transfer, high recovery, and reusability
Electronic supplementary material
ESM 1
(DOCX 123 kb)
Rights and permissions
About this article
Cite this article
Kojidi, M.H., Aliakbar, A. Synthesis of graphene oxide-based poly(p-aminophenol) composite and its application in solid phase extraction of trace amount of Ni(II) from aquatic samples. Environ Monit Assess 191, 145 (2019). https://doi.org/10.1007/s10661-019-7282-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7282-x