Abstract
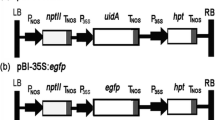
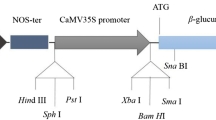
Fusarium rot caused by Fusarium oxysporum f.sp. gladioli (Fog) is one of the most serious diseases of Gladiolus, both in the field and in bulbs in storage. In order to study the mechanisms of pathogenesis of this fungus, we have transformed Fog with Agrobacterium tumefaciens binary vectors containing the hygromycin B phosphotransferase (hph) gene and fluorescence reporter genes EGFP (green), EYFP (yellow) or ECFP (cyan) using the AGL-1 strain of A. tumefaciens. Hygromycin B (100 μg/ml) resistant colonies were observed only when acetosyringone was added to the co-cultivation medium. Transformed colonies are more clearly visible when co-cultivated on cellophane membrane than on Hybond -N+ membrane. Transformed lines were stably maintained through four serial passages on medium containing hygromycin B, and they expressed green, yellow or cyano fluorescence. PCR with hph-specific primers and Southern blotting with an hph-specific probe were positive for HygR lines but not for the untransformed isolate. The cyano fluorescence of the ECFP-transformed isolate was clearly distinguishable from the green autofluorescence of Gladiolus roots, signifying the potential of these lines for further histopathological investigations. Transformed lines will be useful for identifying pathogenicity related genes, screening transgenic resistance, and in studies of host-pathogen interactions.





Similar content being viewed by others
References
Agrios, G. N. (2005). Plant pathology (5th ed.). London: Elsevier Academic Press.
Chen, X., Stone, M., Schlagnhaufer, C., & Romaine, C. P. (2000). A fruiting body tissue method for efficient agrobacterium-mediated transformation of Agaricus bisporus. Applied and Environmental Microbiology, 66(10), 4510–4513.
Cubero, O. F., Crespo, A., Fatehi, J., & Bridge, P. D. (1999). DNA extraction and PCR amplification method suitable for fresh, herbarium and lichenized fungi. Plant Systematics and Evolution, 216(3), 243–249.
Degefu, Y., & Hanif, M. (2003). Agrobacterium tumefaciens-mediated transformation of Helminthosporium turcicum, the maize leafblight fungus. Archives of Microbiology, 180(4), 279–284.
Dhingra, O. D., & Sinclair, J. B. (1995). Basic plant pathology methods (2nd ed.). Boca Raton: CRC/Lewis.
Elmer, W. H. (2006). Efficacy of preplant treatments of gladiolus corms with combinations of acibenzolar-S-methyl and biological or chemical fungicides for suppression of fusarium corm rot [Fusarium oxysporum f. sp. gladioli]. Canadian Journal of Plant Pathology, 28(4), 609–614.
Grimaldi, B., de Raaf, M. A., Filetici, P., Ottonello, S., & Ballario, P. (2005). Agrobacterium-mediated gene transfer and enhanced green fluorescent protein visualization in the mycorrhizal ascomycete Tuber borchii: a first step towards true genetics. Current Genetics, 48(1), 67–74.
Hanif, M., Pardo, A., Gorfer, M., & Raudaskoski, M. (2002). T-DNA transfer and integration in the ectomycorrhizal fungus Suillus bovinus using hygromycin B as a selectable marker. Current Genetics, 41(3), 183–188.
Heimann, M. F., & Worf, G. L. (1997). Gladiolus disorder: Fusarium yellows and bulb rot. WI, USA: Cooperative Extension, University of Wisconsin, University Press. 274 pp.
Jones, R. K., & Jenkins, J. M. (1975). Evaluation of resistance in Gladiolus sp. to Fusarium oxysporum f. sp. gladioli. Phytopathology, 65(4), 481–484.
Kemppainen, M., Circosta, A., Tagu, D., Martin, F., & Pardo, A. G. (2005). Agrobacterium-mediated transformation of the ectomycorrhizal symbiont Laccaria bicolor S238N. Mycorrhiza, 16(1), 19–22.
Khan, M. R., & Mustafa, U. (2005). Corm rot and yellows of Gladiolus and its biomanagement. Phytopathologia Mediterranea, 44(2), 208–215.
Khang, C., Park, S., Rho, H., Lee, Y., & Kang, S. (2006). Agrobacterium tumefaciens-mediated transformation and mutagenesis of filamentous fungi Magnaporthe grisea and Fusarium oxysporum. In K. Wang (Ed.), Agrobacterium protocols (pp. 403–420). Totowa: Humana.
Kilaru, S., Collins, C. M., Hartley, A. J., Bailey, A. M., & Foster, G. D. (2009). Establishing molecular tools for genetic manipulation of the pleuromutilin producing fungus Clitopilus passeckerianus. Applied and Environmental Microbiology, 75(22), 7196–7204.
Kim, H.-S., Park, S.-Y., Lee, S., Adams, E. L., Czymmek, K., & Kang, S. (2011). Loss of cAMP-dependent protein kinase A affects multiple traits important for root pathogenesis by Fusarium oxysporum. Molecular Plant-Microbe Interactions, 24(6), 719–732.
Lagopodi, A. L., Ram, A. F. J., Lamers, G. E. M., Punt, P. J., Van den Hondel, C. A. M. J. J., Lugtenberg, B. J. J., & Bloemberg, G. V. (2002). Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicislycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Molecular Plant-Microbe Interactions, 15(2), 172–179.
Lakshman, D., Pandey, R., Kamo, K., Mitra, A., & Bauchan, G. (2010). Agrobacterium-mediated transformation of Fusarium oxysporum fsp. gladioli to study pathogenesis in Gladiolus. Phytopathology, 100(6).
Lievens, B., Rep, M., & Thomma, B. P. H. J. (2008). Recent developments in the molecular discrimination of formae speciales of Fusarium oxysporum. Pest Management Science, 64(8), 781–788.
Löffler, H. J. M., Straathof, T. P., Van Rubroek, P. C. L., & Roebroeck, E. J. A. (1997). Fusarium resistance in gladiolus: the development of a screening assay. Journal of Phytopathology, 145(11–12), 465–468.
Magie, R. O. (1980). Fusarium diseases of gladioli controlled by inoculation of corms with non-pathogenic Fusaria. Proceedings of the Florida State Horticultural Society, 93, 172–175.
Magie, R. O. (2000). Some Fungi That Attack Gladioli. Farming and Agriculture Series, Library4Farming. (http://science-in-farming.library4farming.org/index.html).
Maniatis, T., Fritsch, E. F., & Sambrook, J. (1982). Molecular cloning: A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
McDonnell, K. (1962). Relationship of pectic enzymes and pathogenicity in the fusarium wilt of tomatoes. Transactions of the British Mycological Society, 45(1), 55–62.
Michielse, C. B., & Rep, M. (2009). Pathogen profile update: Fusarium oxysporum. Molecular Plant Pathology, 10(3), 311–324.
Michielse, C. B., van Wijk, R., Reijnen, L., Cornelissen, B. J. C., & Rep, M. (2009). Insight into the molecular requirements for pathogenicity of Fusarium oxysporum f. sp. lycopersici through large-scale insertional mutagenesis. GenomeBiology.com, 10(1), R4.
Mosquera, G., Giraldo, M. C., Khang, C. H., Coughlan, S., & Valent, B. (2009). Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as Biotrophy-associated secreted proteins in rice blast disease. The Plant Cell, 21(4), 1273–1290.
Mullins, E., Chen, X., Romaine, P., Raina, R., Geiser, D., & Kang, S. (2001). Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology, 91(2), 173–180.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497.
Riaz, T., Khan, S. N., & Javaid, A. (2010). Screening of Gladiolus germplasm for agronomic performance and resistance against corm rot disease. African Journal of Biotechnology, 9(40), 6701–6707.
Roebroeck, E. J. A., & Mes, J. J. (1992). Physiological races and vegetative compatibility groups within Fusarium oxysporum f.sp. gladioli. European Journal of Plant Pathology, 98(1), 57–64.
Schenk, P. K., & Bergman, B. H. H. (1969). Uncommon disease symptoms caused by Fusarium oxysporum in tulips forced in the glasshouse after pre-cooling at 5°C. European Journal of Plant Pathology, 75, 100–104.
Straathof, T. P., Roebroeck, E. J. A., & Löffler, H. J. M. (1998). Studies on Fusarium-Gladiolus interactions. Journal of Phytopathology, 146(2–3), 83–88.
Talhinhas, P., Muthumeenakshi, S., Neves-Martins, J., Oliveira, H., & Sreenivasaprasad, S. (2008). Agrobacterium-mediated transformation and insertional mutagenesis in Colletotrichum acutatum for investigating varied pathogenicity lifestyles. Molecular Biotechnology, 39(1), 57–67. doi:10.1007/s12033-007-9028-1.
Thirugnanasambandam, A., Wright, K. M., Whisson, S. C., Atkins, S. D., & Newton, A. C. (2011). Infection of Rrs1 barley by an incompatible race of the fungus, Rhynchosporium secalis expressing the green fluorescent protein. Plant Pathology, 60(3), 513–521.
Tsuji, G., Fujihara, N., Hirose, C., Tsuge, S., Shiraishi, T., & Kubo, Y. (2003). Agrobacterium tumefaciens-mediated transformation for random insertional mutagenesis in Colletotrichum lagenarium. Journal of General Plant Pathology, 69(4), 230–239.
Ushimaru, T., Terada, H., Tsuboi, K., Kogou, Y., Sakaguchi, A., Tsuji, G., & Kubo, Y. (2010). Development of an efficient gene targeting system in Colletotrichum higginsianum using a non-homologous end-joining mutant and Agrobacterium tumefaciens-mediated gene transfer. Molecular Genetics and Genomics, 284(5), 357–371.
Yang, Y., Cao, T., Yang, J., Howard, R. J., Kharbanda, P. D., & Strelkov, S. E. (2010). Histopathology of internal fruit rot of sweet pepper caused by Fusarium lactis. Canadian Journal of Plant Pathology, 32(1), 86–97.
Zhang, P., Xu, B., Wang, Y., Li, Y., Qian, Z., Tang, S., Huan, S., & Ren, S. (2008). Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in the fungus Penicillium marneffei. Mycological Research, 112(8), 943–949.
Acknowledgements
We thank Prof. Seogchan Kang (Penn State Univ., PA) for supplying the Agrobacterium strains containing the binary vectors, Prof. Robert McGovern (Univ. of Florida, Gainesville, FL) for supplying the F. oxysporum f.sp. gladioli used in this study, Siobhan O’Connor for the Southern analyses, and Chris Pooley (SGIL, USDA-ARS, Beltsville) for help with processing the confocal images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshman, D.K., Pandey, R., Kamo, K. et al. Genetic transformation of Fusarium oxysporum f.sp. gladioli with Agrobacterium to study pathogenesis in Gladiolus . Eur J Plant Pathol 133, 729–738 (2012). https://doi.org/10.1007/s10658-012-9953-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-9953-0