Abstract
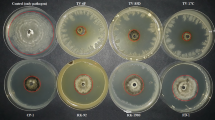
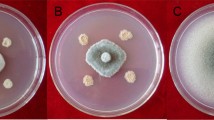
Bacillus subtilis CPA-8, a strain with demonstrated ability to control Monilinia spp. in peaches, was studied to elucidate its mechanisms of antifungal activity. Growth inhibition assays using cell-free supernatants and butanolic extracts showed strong antifungal activities against Monilinia laxa and Monilinia fructicola. By comparison with the reference B. subtilis strains UMAF6614 and UMAF6639, fengycin, iturin and surfactin lipopeptides were identified by thin layer chromatography in butanolic extracts from cell-free supernatants, indicating that antibiosis could be a major factor involved in the biological control ability of CPA-8. TLC-bioautography analysis confirmed the presence of fengycin, iturin and surfactin lipopeptides but strong antifungal activity could be associated only with fengycin lipopeptides. These results were definitively supported by mutagenesis analysis targeted to suppress fengycin biosynthesis by disruption of the B. subtilis fenB gene. By TLC-bioautography analysis it was possible to identify transformants from CPA-8 with reduced or suppressed antifungal activity, and this phenotype was associated with the lack of fengycin bands. Fruit trials confirmed that fengycin-defective mutants and their cell-free supernatants lost their ability to control peach brown rot disease in comparison with CPA-8 wild type strain or Serenade Max®, a commercial formulation based on B. subtilis. Furthermore, population dynamics studies determined that CPA-8 fengycin-deficient mutants survived in wounds in peach fruit equally well as the CPA-8 wild type. Taken together our data indicate that fengycin-like lipopeptides play a major role in the biological control potential of B. subtilis CPA-8 against peach brown rot.



Similar content being viewed by others
References
Arrebola, E., Jacobs, R., & Korsten, L. (2010). Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. Journal of Applied Microbiology, 108, 386–395.
Casals, C., Teixidó, N., Viñas, I., Cambray, J., & Usall, J. (2010a). Control of Monilinia spp. on stone fruit by curing treatments. Part II: The effect of host and Monilinia spp. variables on curing efficacy. Postharvest Biology and Technology, 56, 26–30.
Casals, C., Teixidó, N., Viñas, I., Llauradó, S., & Usall, J. (2010b). Control of Monilinia spp. on stone fruit by curing treatments. Part I: the effect of temperature, exposure time and relative humidity on curing efficacy. Postharvest Biology and Technology, 56, 19–25.
Casals, C., Teixidó, N., Viñas, I., Silvera, E., Lamarca, N., & Usall, J. (2010c). Combination of hot water, Bacillus subtilis CPA-8 and sodium bicarbonate treatments to control postharvest brown rot on peaches and nectarines. European Journal of Plant Pathology, 128, 51–63.
Casals, C., Viñas, I., Torres, R., Griera, C., & Usall, J. (2010d). Effect of temperature and water activity on in vitro germination of Monilinia spp. Journal of Applied Microbiology, 108, 47–54.
De Cal, A., & Melgarejo, P. (1999). Effects of long-wave UV light on Monilinia growth and identification of species. Plant Disease, 83, 62–65.
De Cal, A., Gell, I., Usall, J., Viñas, I., & Melgarejo, P. (2009). First report of brown rot caused by Monilinia fructicola in peach orchards in Ebro Valley, Spain. Plant Disease, 93, 763.
Deleu, M., Paquot, M., & Nylander, T. (2005). Fengycin interaction with lipid monolayers at the air–aqueous interface—implications for the effect of fengycin on biological membranes. Journal of Colloid and Interface Science, 283, 358–365.
Droby, S., Wisniewski, M., Macarisin, D., & Wilson, C. (2009). Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biololgy and Technology, 52, 137–145.
Duitman, E. H., Hamoen, L. W., Rembold, M., Venema, G., Seitz, H., Saenger, W., et al. (1999). The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proceedings of the National Academy of Sciences of the United States of America, 96, 13294–13299.
EPPO A2 List of pests recommended for regulation as quarantine pests (version 2010). Retrieved June 27, 2011 from http://www.eppo.org/QUARANTINE/listA2.htm.
Fravel, D. R. (2005). Commercialization and implementation of biocontrol. Annual Review of Phytopathology, 43, 337–359.
González-Sánchez, M. A., Pérez-Jiménez, R. M., Pliego, C., Ramos, C., De Vicente, A., & Cazorla, F. M. (2010). Biocontrol bacteria selected by a direct plant protection strategy against avocado white root rot show antagonism as a prevalent trait. Journal of Applied Microbiology, 109, 65–78.
Gueldner, R. C., Reilly, C. C., Pusey, P. L., Costello, C. E., Arrendale, R. F., Cox, R. H., et al. (1988). Isolation and identification of iturins as antifungal peptides in biological control of peach brown rot with Bacillus subtilis. Journal of Agricultural and Food Chemistry, 36, 366–370.
Heungens, K., & Parke, J. L. (2001). Postinfection biological control of oomycete pathogens of pea by Burkholderia cepacia AMMDR1. Phytopathology, 91, 383–391.
Hokeberg, M., Wright, S. A. I., Svensson, M., Lundgren, L. N., & Gerhardson, B. (1998). Mutants of Pseudomonas chlororaphis defective in the production of an antifungal metabolite express reduced biocontrol activity (Paper presented at the 7th International Congress of Plant Pathology, Edinburgh).
Jacques, P., Hbid, C., Vanhentreyck, F., Destain, J., Bare, G., Razafindralambo, H., et al. (1994). Quantitative and qualitative study of the production of broad-spectrum antifungal lipopeptides from Bacillus subtilis S499 (Paper presented at 6th European Congress on Biotechnology, Florence).
Janisiewicz, W., & Korsten, L. (2002). Biological control of postharvest diseases of fruit. Annual Review of Phytopathology, 40, 411–441.
Larena, I., Torres, R., De Cal, A., Liñan, M., Melgarejo, P., Domenichini, P., et al. (2005). Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biological Control, 32, 305–310.
Liu, J., Zhou, T., He, D., Li, X. Z., Wu, H., Liu, W., et al. (2011). Functions of lipopeptides bacillomycin D and fengycin in antagonism of Bacillus amyloliquefaciens C06 towards Monilinia fructicola. Journal of Molecular Microbiology and Biotechnology, 20, 43–52.
Lu, J. Y., Stevens, C., Khan, V. A., Kabne, M., & Wilson, C. L. (1991). The effect of ultraviolet irradiation on shelf-life and ripening of peaches and apples. Journal of Food Quality, 14, 299–305.
Magnet-Dana, R., Thimon, L., Peypoux, F., & Ptak, M. (1992). Surfactin/iturin A interactions may explain the synergistic effect of surfactin on the biological properties of iturin A. Biochimie, 74, 1047–1051.
Mari, M., Gregori, R., & Donati, I. (2004). Postharvest control of Monilinia laxa and Rhizopus stolonifer in stone fruit by peracetic acid. Postharvest Biology and Technology, 33, 319–325.
Mari, M., Leoni, O., Bernardi, R., Neri, F., & Palmieri, S. (2008). Control of brown rot on stonefruit by synthetic and glucosinolate-derived isothiocyanates. Postharvest Biology and Technology, 47, 61–67.
Mari, M., Torres, R., Casalini, L., Lamarca, N., Mandrin, J. F., Lichou, J., et al. (2007). Control of post-harvest brown rot on nectarine by Epicoccum nigrum and physico-chemical treatments. Journal of the Science of Food and Agriculture, 87, 1271–1277.
McKeen, C. D., Reilly, C. C., & Pusey, P. L. (1986). Production and partial characterization of antifungal substances antagonistic to Monilinia fructicola from Bacillus subtilis. Phytopathology, 76, 136–139.
Moyne, A. L., Cleveland, T. E., & Tuzun, S. (2004). Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiology Letters, 234, 43–49.
Ongena, M., & Jacques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends in Microbiology, 16, 115–125.
Ongena, M., Henry, G., & Thonart, P. (2009). The roles of cyclic lipopeptides in the biocontrol activity of Bacillus subtilis. In U. Gisi, L. Chet, M. L. Gullino (Eds.), Recent developments in management of plant diseases, plant pathology in the 21st century. (Paper presented at the 9th International Congress of Plant Pathology, Berlin).
Ongena, M., Jacques, P., Touré, Y., Destain, J., Jabrane, A., & Thonart, P. (2005). Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Applied Microbiology and Biotechnology, 69, 29–38.
Palou, L., Smilanick, J. L., & Crisosto, C. H. (2009). Evaluation of food additives as alternative or complementary to conventional fungicides for the control of major postharvest diseases of stone fruit. Journal of Food Protection, 72, 1037–1046.
Pusey, P. L., & Wilson, C. L. (1984). Postharvest biological control of stone fruit brown rot by Bacillus subtilis. Plant Disease, 68, 753–756.
Raaijmakers, J. M., Vlami, M., & de Souza, J. T. (2002). Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek International Journal of General and Molecular Microbiology, 81, 537–547.
Razafindralambo, H., Paquot, M., Hbid, C., Jacques, P., Destain, J., & Thonart, P. (1993). Purification of antifungal lipopeptides by reversed-phase high performance liquid chromatography. Journal of Chromatography, 639, 81–85.
Romero, D., de Vicente, A., Rakotoaly, R. H., Dufour, S. E., Veening, J. W., Arrebola, E., et al. (2007). The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis towards Podosphaera fusca. Molecular Plant–Microbe Interactions, 20, 430–440.
Romero, D., Pérez-García, A., Rivera, M. E., Cazorla, F. M., & de Vicente, A. (2004). Isolation and evaluation of antagonistic bacteria towards the cucurbit powdery mildew fungus Podosphaera fusca. Applied Microbiology and Biotechnology, 64, 263–269.
Romero, D., Pérez-García, A., Veening, J. W., de Vicente, A., & Kuiper, O. P. (2006). Transformation of undomesticated strains of Bacillus subtilis by protoplast electroporation. Journal of Microbiological Methods, 66, 556–559.
Shoda, M. (2000). Bacterial control of plant diseases. Journal of Bioscience and Bioengineering, 89, 515–521.
Silo-Suh, L. A., Lethbridge, B. J., Raffel, S. I., He, H. Y., Clardy, J., & Handelsman, J. (1994). Biological-activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Applied and Environmental Microbiology, 60, 2023–2030.
Stein, T. (2005). Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology, 56, 845–857.
Touré, Y., Ongena, M., Jacques, P., Guiro, A., & Thonart, P. (2004). Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. Journal of Applied Microbiology, 96, 1151–1160.
Vanittanakom, N., Loeffer, W., Koch, U., & Jung, G. (1986). Fengycin—a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. The Journal of Antibiotics, 39, 888–901.
Wilson, C. L., & Pusey, P. L. (1985). Potential for biological control of postharvest plant diseases. Plant Disease, 69, 375–378.
Yánez-Mendizábal, V., Usall, J., Viñas, I., Casals, C., Marín, S., Solsona, C., et al. (2011). Potential of a new strain of Bacillus subtilis CPA-8 to control the major postharvest diseases of fruit. Biocontrol Science and Technology, 21, 409–426.
Yoshida, S., Hiradate, S., Tsukamoto, T., Hatakeda, K., & Shirata, A. (2001). Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology, 91, 181–187.
Zhang, D., Spadaro, D., Garibaldi, A., & Gullino, M. L. (2010). Selection and evaluation of new antagonists for their efficacy against postharvest brown rot of peaches. Postharvest Biology and Technology, 55, 174–181.
Acknowledgements
The authors are grateful to the Spanish Government, Ministerio de Asuntos Exteriores de Cooperación, Agencia Española de Cooperación Internacional para el Desarrollo (AECID) for grant scholarship 0000308852 (V. Yánez-Mendizábal), Plan Nacional de I+D+I of the Ministerio de Ciencia e Innovación (AGL2007-65340-CO2-01), co-financed with FEDER funds (European Union), the REDBIO European Project and the UdL (University of Lleida) Organic Project 2009 for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yánez-Mendizábal, V., Zeriouh, H., Viñas, I. et al. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur J Plant Pathol 132, 609–619 (2012). https://doi.org/10.1007/s10658-011-9905-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-011-9905-0