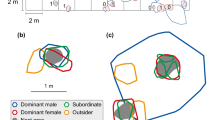
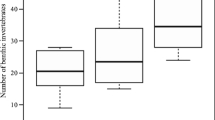
Abstract
The ‘benefits of philopatry’ hypothesis states that helpers in cooperatively breeding species derive higher benefits from remaining home, instead of dispersing and attempting to breed independently. We tested experimentally whether dispersal options influence dispersal propensity in the cooperatively breeding Lake Tanganyika cichlids Neolamprologus pulcher and N. savoryi. Cooperative groups of these fishes breed in densely packed colonies, surrounded by unoccupied, but apparently suitable breeding habitat. Breeding inside colonies and living in groups seems to benefit individuals, for example by early detection and deterrence of predators. We show that despite a slight preference of both species for habitat with a higher stone cover, 40% of the preferred habitat remained unoccupied. On average, the colonies contained a higher number of (1) predators of adults, juveniles and eggs, (2) shelter competitors, and (3) other species including potential food competitors, compared to the outside colony habitat. Apparently, habitat differences cannot explain why these cichlids breed in colonies. Accordingly, dispersal may not be limited by a lack of suitable breeding shelters, but by the relatively higher risk of establishing an outside- compared to a within-colony breeding territory. To test whether cichlids prefer within- to outside-colony breeding territories, we provided breeding shelters inside the colony and at the colony edge and studied helper dispersal. As expected, significantly more shelters were occupied within the colony compared to the edge. New breeding pairs with several helpers occupied these shelters. We conclude that although breeding habitat is plentiful outside the colonies, helpers delay dispersal to obtain a higher quality breeding position within the group or colony eventually, or they disperse in groups. Our results suggest that (1) group augmentation and Allee effects are generally important for dispersal decisions in cooperatively breeding cichlids, consistent with the ‘benefits of philopatry hypothesis’, and (2) habitat saturation cannot fully explain delayed dispersal in these species.





Similar content being viewed by others
References
Allee WC (1951) The social life of animals. Beacon Press, Boston
Arnold KE (2000) Group mobbing behaviour and nest defence in a cooperatively breeding Australian bird. Ethology 106:385–393
Balshine S, Leach B, Neat F, Reid H, Taborsky M, Werner N (2001) Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav Ecol Sociobiol 50:134–140
Balshine-Earn S, Neat FC, Reid H, Taborsky M (1998) Paying to stay or paying to breed? Field evidence for direct benefits of hel** behavior in a cooperatively breeding fish. Behav Ecol 9:432–438
Bergmüller R, Heg D, Peer K, Taborsky M (2005a) Extended safe havens and between-group dispersal of helpers in a cooperatively breeding cichlid. Behaviour 142:1643–1667
Bergmüller R, Heg D, Taborsky M (2005b) Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc Royal Soc Lond B 272:325–331
Booth DJ (1992) Larval settlement patterns and preferences by domino damselfish Dascyllus albisella Gill. J Exp Mar Biol Ecol 155:85–104
Booth DJ, Wellington G (1998) Settlement preferences in coral-reef fishes: effects on patterns of adult and juvenile distributions, individual fitness and population structure. Austr J Ecol 23:274–279
Brichard P (1997) Atlas der Tanganjikasee cichliden, Band 1. Bede-Verlag, Ruhmannsfelden
Brichard P (1999) Atlas der Tanganjikasee cichliden, Band 2. Bede-Verlag, Ruhmannsfelden
Brouwer L, Heg D, Taborsky M (2005) Experimental evidence for helper effects in a cooperatively breeding cichlid. Behav Ecol 16:667–673
Buston P (2003) Forcible eviction and prevention of recruitment in the clown anemonefish. Behav Ecol 14:576–582
Choe JC, Crespi BJ (1997) The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge
Courchamp F, Clutton-Brock T, Grenfell B (1999a) Inverse density dependence and the Allee effect. Trends Ecol Evol 14:405–410
Courchamp F, Grenfell B, Clutton-Brock T (1999b) Population dynamics of obligate cooperators. Proc Royal Soc Lond B 266:557–563
Courchamp F, Grenfell BT, Clutton-Brock TH (2000) Impact of natural enemies on obligately cooperative breeders. Oikos 91:311–322
Crawley MJ (2002) Statistical computing. An introduction to data analysis using S-plus. Wiley, Chichester
Danchin E, Heg D, Doligez B (2001) Public information and breeding habitat selection. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal: individual, population, and community. Oxford University Press, Oxford, pp 243–258
Dierkes P, Heg D, Taborsky M, Skubic E, Achmann R (2005) Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol Lett 8:968–975
Duplessis MA (1992) Obligate cavity roosting as a constraint on dispersal of green (red-billed) woodhoopoes – consequences for philopatry and the likelihood of inbreeding. Oecologia 90:205–211
Emlen ST (1995) An evolutionary theory of the family. Proc Natl Acad Sci U S A 92:8092–8099
Field J, Shreeves G, Sumner S (1999) Group size, queuing and hel** decisions in facultatively eusocial hover wasps. Behav Ecol Sociobiol 45:378–385
Gashagaza MM (1988) Feeding activity of a Tanganyikan cichlid fish Lamprologus brichardi. Afr Study Monogr 9:1–9
Goldizen AW, Putland DA, Robertson KA (2002) Dispersal strategies in Tasmanian native hens (Gallinula mortierii). Behav Ecol 13:328–336
Greene CM, Stamps JA (2001) Habitat selection at low population densities. Ecology 82:2091–2100
Hatchwell BJ, Komdeur J (2000) Ecological constraints, life history traits and the evolution of cooperative breeding. Anim Behav 59:1079–1086
Heg D, Bachar Z (2006) Cooperative breeding in the Lake Tanganyika cichlid Julidochromis ornatus. Environ Biol Fish 76:265–281
Heg D, Bachar Z, Brouwer L, Taborsky M (2004) Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc Royal Soc Lond B271:2367–2374
Heg D, Bachar Z, Taborsky M (2005a) Cooperative breeding and group structure in the Lake Tanganyika cichlid Neolamprologus savoryi. Ethology 111:1017–1043
Heg D, Brouwer L, Bachar Z, Taborsky M (2005b) Large group size yields group stability in the cooperatively breeding cichlid Neolamprologus pulcher. Behaviour 142:1615–1641
Heg D, Bergmüller R, Bonfils D, Otti O, Bachar Z, Burri R, Heckel G, Taborsky M (2006) Cichlids do not adjust reproductive skew to the availability of independent breeding options. Behav Ecol 17:419–429
Heinsohn R, Dunn P, Legge S, Double M (2000) Coalitions of relatives and reproductive skew in cooperatively breeding white-winged choughs. Proc Royal Soc Lond B 267:243–249
Koenig WD, Dickinson JL (2004) Ecology and evolution of cooperative breeding in birds. Cambridge University Press, Cambridge
Koenig WD, Pitelka FA, Carmen WJ, Mumme RL, Stanback MT (1992) The evolution of delayed dispersal in cooperative breeders. Quart Rev Biol 67:111–150
Kohler U (1998) Zur Struktur und Evolution des Sozialsystems von Neolamprologus multifasciatus (Cichlidae, Pisces), dem kleinsten Schneckenbuntbarsch des Tanganjikasees. Shaker Verlag, Aachen
Komdeur J (1992) Importance of habitat saturation and territory quality for evolution of cooperative breeding in the seychelles warbler. Nature 358:493–495
Komdeur J (1994) Experimental evidence for hel** and hindering by previous offspring in the cooperative-breeding seychelles warbler Acrocephalus sechellensis. Behav Ecol Sociobiol 34:175–186
Komdeur J, Edelaar P (2001) Male seychelles warblers use territory budding to maximize lifetime fitness in a saturated environment. Behav Ecol 12:706–715
Konings A (1998) Tanganyika cichlids in their natural habitat. Cichlid, El Paso
Kondo T (1986) Feeding habits of Lamprologus savoryi (Teleostei: Cichlidae) with reference to its social behaviour. Physiol Ecol 23:1–15
Kudo SI, Ishibashi E, Makino S (1995) Reproductive and subsocial behaviour in the ovoviviparous leaf beetle Gonioctena sibirica (Coleoptera: Chrysomelidae). Ecol Entomol 20:367–373
Kurki H, Vuorinen I, Bosma E, Bwebwa D (1999) Spatial and temporal changes in copepod zooplankton communities of Lake Tanganyika. Hydrobiol 407:105–114
Kuwamura T (1997) The evolution of parental care and mating systems among Tanganyikan cichlids. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in Lake Tanganyika. Kyoto University Press, Kyoto, pp 59–86
Ligon JD, Ligon SH, Ford HA (1991) An experimental study of the bases of male philopatry in the cooperatively breeding superb fairy-wren Malurus cyaneus. Ethology 87:134–148
Limberger D (1983) Pairs and harems in a cichlid fish, Lamprologus brichardi. Z Tierpsychol 62:115–144
Macedo RH, Bianchi CA (1997) Communal breeding in tropical guira cuckoos Guira guira: sociality in the absence of a saturated habitat. J Avian Biol 28:207–215
Pruett-Jones SG, Lewis MJ (1990) Sex ratio and habitat limitation promote delayed dispersal in superb fairy-wrens. Nature 348:541–542
Rasa OAE (1987) The dwarf mongoose: a study of behavior and social structure in relation to ecology in a small, social carnivore. Adv Study Behav 17:121–163
Skubic E, Taborsky M, McNamara JM, Houston AI (2004) When to parasitize? A dynamic optimization model of reproductive strategies in a cooperative breeder. J Theor Biol 227:487–501
Stacey PB, Koenig WD (1990) Cooperative breeding in birds. Cambridge University Press, Cambridge
Stacey PB, Ligon JD (1987) Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. Am Nat 130:654–676
Stacey PB, Ligon JD (1991) The benefits-of-philopatry hypothesis for the evolution of cooperative breeding – variation in territory quality and group size effects. Am Nat 137:831–846
Stamps JA (1988) Conspecific attraction and aggregation in territorial species. Am Nat 131:329–347
Stamps JA (2001) Habitat selection by dispersers: integrating proximate and ultimate approaches. In: Clobert J, Danchin E, Dhondt AA, Nichols JD (eds) Dispersal: individual, population, and community. Oxford University Press, Oxford, pp 230–242
Stephens PA, Sutherland WJ (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol 14:401–405
Stiver KA, Dierkes P, Taborsky M, Balshine S (2004) Dispersal patterns and status change in a co-operatively breeding cichlid: evidence from microsatellite analyses and behavioural observations. J Fish Biol 65:91–105
Stiver KA, Fitzpatrick J, Desjardins JK, Balshine S (2006) Sex differences in rates of territory joining and inheritance in a cooperatively breeding cichlid fish. Anim Behav 71:449–456
Sweatman HPA (1983) Influence of conspecifics on choice of settlement sites by larvae of two pomacentrid fishes (Dascyllus aruanus and D. reticulatus) on coral reefs. Mar Biol 75:222–229
Sweatman HPA (1985) The influence of adults of some coral reef fishes on larval recruitment. Ecol Monogr 55:469–485
Taborsky M (1984) Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim Behav 32:1236–1252
Taborsky M (1994) Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv Study Behav 23:1–100
Taborsky M, Limberger D (1981) Helpers in fish. Behav Ecol Sociobiol 8:143–145
Tibbetts EA, Reeve HK (2003) Benefits of foundress associations in the paper wasp Polistes dominulus: increased productivity and survival, but no assurance of fitness returns. Behav Ecol 14:510–514
Werner NY, Balshine S, Leach B, Lotem A (2003) Hel** opportunities and space segregation in cooperatively breeding cichlids. Behav Ecol 14:749–756
Zar JH (1984) Biostatistical analysis. Prentice Hall, Englewood Cliffs
Acknowledgments
We thank C. Kapasa, H. Phiri, R. Shapola, L. Makasa, D. Sinyinza and C. Lukwesa from the Department of Fisheries, Zambia Ministry of Agriculture and Co-operatives for their continuous support of our project. We thank the members of the Lake Tanganyika Diving Expeditions 2002 and 2003 for their assistance. We are grateful to Rolf Eggler, Susanne Maurer and Peter Stettler for logistical support and Ralph Bergmüller and anonymous reviewers for comments on the manuscript. The project was supported by the Swiss National Science Foundation (SNF grant 3100-064396 to M.T.). D.H. is supported by SNF grant 3100A0-108473. The experiments conducted in this study comply with the current laws of the country, Zambia, in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heg, D., Heg-Bachar, Z., Brouwer, L. et al. Experimentally induced helper dispersal in colonially breeding cooperative cichlids. Environ Biol Fish 83, 191–206 (2008). https://doi.org/10.1007/s10641-007-9317-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-007-9317-3