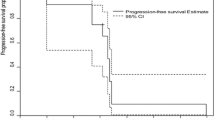
Summary
Objectives Heat Shock Protein 90 (HSP90) is a molecular chaperone that stabilizes many oncogenic proteins. HSP90 inhibitors may sensitize tumors to cytotoxic agents by causing client protein degradation. Gemcitabine, which has modest activity in pancreas cancer, activates Chk1, a client protein of HSP90. This phase II trial was designed to determine whether 17AAG could enhance the clinical activity of gemcitabine through degradation of Chk1 in patients with stage IV pancreatic cancer. Methods A multicenter, prospective study combining gemcitabine and 17AAG enrolled patients with stage IV pancreatic adenocarcinoma, adequate liver and kidney function, ECOG performance status 0–2, and no prior chemotherapy for metastatic disease. The primary goal was to achieve a 60 % overall survival at 6 months. Sixty-six patients were planned for accrual, with an interim analysis after 25 patients enrolled. Results After a futility analysis to achieve the endpoint, accrual was halted with 21 patients enrolled. No complete or partial responses were seen. Forty percent of patients were alive at 6 months. Median overall survival was 5.4 months. Tolerability was moderate, with 65 % of patients having ≥ grade 3 adverse events (AE), and 15 % having grade 4 events. Conclusions The lack of clinical activity suggests that targeting Chk1 by inhibiting HSP90 is not important in pancreatic cancer sensitivity to gemcitabine alone. Further studies of HSP90 targeted agents with gemcitabine alone are not warranted.

Similar content being viewed by others
References
Cancer facts and figures (2013). American Cancer Society, Atlanta
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18):1691–1703. doi:10.1056/NEJMoa1304369
Pratt WB, Toft DO (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18(3):306–360
An WG, Schnur RC, Neckers L, Blagosklonny MV (1997) Depletion of p185erbB2, Raf-1 and mutant p53 proteins by geldanamycin derivatives correlates with antiproliferative activity. Cancer Chemother Pharmacol 40(1):60–64
Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG et al (1995) Inhibition of the oncogene product p185erbB-2 in vitro and in vivo by geldanamycin and dihydrogeldanamycin derivatives. J Med Chem 38(19):3806–3812
Xu W, Yuan X, Jung YJ, Yang Y, Basso A, Rosen N, Chung EJ, Trepel J, Neckers L (2003) The heat shock protein 90 inhibitor geldanamycin and the ErbB inhibitor ZD1839 promote rapid PP1 phosphatase-dependent inactivation of AKT in ErbB2 overexpressing breast cancer cells. Cancer Res 63(22):7777–7784
Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Skele KL, Hoffman JP, Testa JR (2002) Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem 87(4):470–476
Semba S, Moriya T, Kimura W, Yamakawa M (2003) Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas 26(3):250–257
Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE (1994) Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet 8(1):27–32. doi:10.1038/ng0994-27
Karnitz LM, Flatten KS, Wagner JM, Loegering D, Hackbarth JS, Arlander SJ, Vroman BT, Thomas MB, Baek YU, Hopkins KM, Lieberman HB, Chen J, Cliby WA, Kaufmann SH (2005) Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol Pharmacol 68(6):1636–1644. doi:10.1124/mol.105.012716
Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM (2003) Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem 278(52):52572–52577. doi:10.1074/jbc.M309054200
Hubbard J, Erlichman C, Toft DO, Qin R, Stensgard BA, Felten S, Ten Eyck C, Batzel G, Ivy SP, Haluska P (2011) Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors. Investig New Drugs 29(3):473–480. doi:10.1007/s10637-009-9381-y
Kaplan E, Meier P (1958) Nonparametric estimation for incomplete observations. J Am Stat Assoc 53:457–481
Hendrickson AE, Oberg AL, Glaser G, Camoriano JK, Peethambaram PP, Colon-Otero G, Erlichman C, Ivy SP, Kaufmann SH, Karnitz LM, Haluska P (2012) A phase II study of gemcitabine in combination with tanespimycin in advanced epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol 124(2):210–215. doi:10.1016/j.ygyno.2011.10.002
Kaufmann SH, Karp JE, Litzow MR, Mesa RA, Hogan W, Steensma DP, Flatten KS, Loegering DA, Schneider PA, Peterson KL, Maurer MJ, Smith BD, Greer J, Chen Y, Reid JM, Ivy SP, Ames MM, Adjei AA, Erlichman C, Karnitz LM (2011) Phase I and pharmacological study of cytarabine and tanespimycin in relapsed and refractory acute leukemia. Haematologica 96(11):1619–1626. doi:10.3324/haematol.2011.049551
Kim HR, Kang HS, Kim HD (1999) Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life 48(4):429–433. doi:10.1080/713803536
McCollum AK, Lukasiewicz KB, Teneyck CJ, Lingle WL, Toft DO, Erlichman C (2008) Cisplatin abrogates the geldanamycin-induced heat shock response. Mol Cancer Ther 7(10):3256–3264. doi:10.1158/1535-7163.MCT-08-0157
Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith DF (1996) A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones 1(4):237–250
Whitesell L, Bagatell R, Falsey R (2003) The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets 3(5):349–358
Winklhofer KF, Reintjes A, Hoener MC, Voellmy R, Tatzelt J (2001) Geldanamycin restores a defective heat shock response in vivo. J Biol Chem 276(48):45160–45167. doi:10.1074/jbc.M104873200
Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, Whitesell L (2000) Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin Cancer Res 6(8):3312–3318
Burris HA 3rd, Berman D, Murthy B, Jones S (2011) Tanespimycin pharmacokinetics: a randomized dose-escalation crossover phase 1 study of two formulations. Cancer Chemother Pharmacol 67(5):1045–1054. doi:10.1007/s00280-010-1398-6
Hong DS, Banerji U, Tavana B, George GC, Aaron J, Kurzrock R (2013) Targeting the molecular chaperone heat shock protein 90 (HSP90): lessons learned and future directions. Cancer Treat Rev 39(4):375–387. doi:10.1016/j.ctrv.2012.10.001
Goldman JW, Raju RN, Gordon GA, El-Hariry I, Teofilivici F, Vukovic VM, Bradley R, Karol MD, Chen Y, Guo W, Inoue T, Rosen LS (2013) A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer 13:152. doi:10.1186/1471-2407-13-152
Hong D, Said R, Falchook G, Naing A, Moulder S, Tsimberidou AM, Galluppi G, Dakappagari N, Storgard C, Kurzrock R, Rosen LS (2013) Phase I study of BIIB028, a selective heat shock protein 90 inhibitor, in patients with refractory metastatic or locally advanced solid tumors. Clin Cancer Res 19(17):4824–4831. doi:10.1158/1078-0432.CCR-13-0477
Sessa C, Shapiro GI, Bhalla KN, Britten C, Jacks KS, Mita M, Papadimitrakopoulou V, Pluard T, Samuel TA, Akimov M, Quadt C, Fernandez-Ibarra C, Lu H, Bailey S, Chica S, Banerji U (2013) First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res 19(13):3671–3680. doi:10.1158/1078-0432.CCR-12-3404
Funding disclosure
Supported by P50 SPORE CA102701, N01-CM-2011-00099, CA15083, Georgeson Professorship Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedersen, K.S., Kim, G.P., Foster, N.R. et al. Phase II trial of gemcitabine and tanespimycin (17AAG) in metastatic pancreatic cancer: a Mayo Clinic Phase II Consortium study. Invest New Drugs 33, 963–968 (2015). https://doi.org/10.1007/s10637-015-0246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0246-2



