Abstract
Background
Toll-like receptors (TLRs) are key players in innate immunity and modulation of TLR signaling has been demonstrated to profoundly affect proliferation and growth in different types of cancer. However, the role of TLRs in human intrahepatic cholangiocarcinoma (ICC) pathogenesis remains largely unexplored.
Aims
We set out to determine if TLRs play any role in ICCs which could potentially make them useful treatment targets.
Methods
Tissue microarrays containing samples from 9 human ICCs and normal livers were examined immunohistochemically for TLR4, TLR7, and TLR9 expression. Proliferation of human ICC cell line HuCCT1 was measured by MTS assay following treatment with CpG-ODN (TLR9 agonist), imiquimod (TLR7 agonist), chloroquine (TLR7 and TLR9 inhibitor) and IRS-954 (TLR7 and TLR9 antagonist). The in vivo effects of CQ and IRS-954 on tumor development were also examined in a NOD-SCID mouse xenograft model of human ICC.
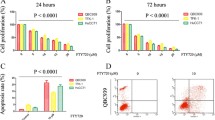
Results
TLR4 was expressed in all normal human bile duct epithelium but absent in the majority (60%) of ICCs. TLR7 and TLR9 were expressed in 80% of human ICCs. However, TLR7 was absent in all cases of normal human bile duct epithelium and only one was TLR9 positive. HuCCT1 cell proliferation in vitro significantly increased following IMQ or CpG-ODN treatment (P < 0.03 and P < 0.002, respectively) but decreased with CQ (P < 0.02). In the mouse xenograft model there was significant reduction in size of tumors from CQ and IRS-954 treated mice compared to untreated controls.
Conclusion
TLR7 and TLR9 should be further explored for their potential as actionable targets in the treatment of ICC.












Similar content being viewed by others
References
Charbel H, Al-Kawas FH. Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep 2011;13:182–187.
Altaee MY, Johnson PJ, Farrant JM, Williams R. Etiologic and clinical characteristics of peripheral and hilar cholangiocarcinoma. Cancer 1991;68:2051–2055.
Shaib Y, El-Serag HB. The Epidemiology of Cholangiocarcinoma. Semin Liver Dis 2004;24:115–125.
Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:221–232.
Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:101–104.
Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist 2016;21:594–599.
Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69–76.
Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg 2006;13:537–542.
Yano Y, Yamamoto J, Kosuge T et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol 2003;33:283–287.
Woo HG, Lee JH, Yoon JH et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res 2010;70:3034–3041.
Jeon J, Maeng LS, Bae YJ, Lee EJ, Yoon YC, Yoon N. Comparing clonality between components of combined hepatocellular carcinoma and cholangiocarcinoma by targeted sequencing. Cancer Genomics Proteomics 2018;15:291–298.
Zhao Q, Yu WL, Lu XY et al. Combined hepatocellular and cholangiocarcinoma originating from the same clone: a pathomolecular evidence-based study. Chin J Cancer 2016;35:82.
Fujii H, Zhu XG, Matsumoto T et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol 2000;31:1011–1017.
Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer 2017;16:4.
Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol 2007;7:1271–1285.
Cen X, Liu S, Cheng K. The role of toll-like receptor in inflammation and tumor immunity. Front Pharmacol 2018;9:878.
Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol 2018;59:391–412.
Basith S, Manavalan B, Yoo TH et al. Roles of toll-like receptors in Cancer: a double-edged sword for defense and offense. Arch Pharm Res 2012;35:1297–1316.
Harada K, Ohira S, Isse K et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Investig 2003;83:1657–1667.
Liu B, Yan S, Jia Y, Ma J et al. TLR2 promotes human intrahepatic cholangiocarcinoma cell migration and invasion by modulating NF-κB pathway-mediated inflammatory responses. FEBS J 2016;283:3839–3850.
Matsushita H, Miyake Y, Takaki A et al. TLR4, TLR9, and NLRP3 in biliary epithelial cells of primary sclerosing cholangitis: Relationship with clinical characteristics. J Gastroenterol Hepatol 2015;30:600–608.
Seki E, De Minicis S, Osterreicher CH et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007;13:1324–1332.
Mohamed F, Al-Jehani R, Minogue S et al. Effect of toll-like receptor 7 and 9 targeted therapy to prevent the development of hepatocellular carcinoma. Liver Int 2015;35:1063–1076.
Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol 2011;25:609579.
Hao B, Chen Z, Bi B et al. Role of TLR4 as a prognostic factor for survival in various cancers: a meta-analysis. Oncotarget 2018;9:13088–13099.
Li J, Yang F, Wei F, Ren X. The role of toll-like receptor 4 in tumor microenvironment. Oncotarget 2017;8:66656–66667.
Shetab Boushehri MA, Lamprecht A. TLR4-based immunotherapeutics in cancer: a review of the achievements and shortcomings. Mol Pharm 2018;15:4777–4800.
Benias PC, Gopal K, Bodenheimer H Jr, Theise ND. Hepatic expression of toll-like receptors 3, 4, and 9 in primary biliary cirrhosis and chronic hepatitis C. Clin Res Hepatol Gastroenterol 2012;36:448–454.
Ninlawan K, O’Hara SP, Splinter PL et al. Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 up regulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int 2010;59:616–621.
Pak JH, Kim IK, Kim SM et al. Induction of cancer-related microRNA expression profiling using excretory-secretory products of Clonorchis sinensis. Parasitol Res 2014;113:4447–4455.
Bahk YY, Pak JH. Toll-like receptor-mediated free radical generation in Clonorchis sinensis excretory-secretory product-treated cholangiocarcinoma cells. Korean J Parasitol 2016;54:679–684.
Gatti G, Quintar AA, Andreani V et al. Expression of Toll-like receptor 4 in the prostate gland and its association with the severity of prostate cancer. Prostate 2009;69:1387–1397.
Yu L, Wang L, Li M, Zhong J, Wang Z, Chen S. Expression of Toll-like receptor 4 is down-regulated during progression of cervical neoplasia. Cancer Immunol Immunother 2010;59:1021–1028.
Paarnio K, Väyrynen S, Klintrup K et al. Divergent expression of bacterial wall sensing toll-like receptors 2 and 4 in colorectal cancer. World J Gastroenterol 2017;23:4831–4838.
Block MS, Vierkant RA, Rambau PF et al. MyD88 and TLR4 expression in epithelial ovarian cancer. Mayo Clin Proc 2018;93:307–320.
Pandey N, Chauhan A, Jain N. TLR4 polymorphisms and expression in solid cancers. Mol Diagn Ther. 2018 (Epub ahead of print). https://doi.org/10.1007/s40291-018-0361-9.
Wang Q, Zhang X, **ao T, Pan C, Liu X, Zhao Y. Prognostic role of Toll-like receptors in cancer: a meta-analysis. Ther Clin Risk Manag 2018;14:1323–1330.
Chatterjee S, Crozet L, Damotte D et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res 2014;74:5008–5018.
Ochi A, Graffeo CS, Zambirinis CP et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Investig 2012;122:4118–4129.
Zhang Y, Wang Q, Ma A, Li Y, Li R, Wang Y. Functional expression of TLR9 in esophageal cancer. Oncol Rep. 2014;31:2298–2304.
Xu L, Wen Z, Zhou Y et al. MicroRNA-7-regulated TLR9 signaling-enhanced growth and metastatic potential of human lung cancer cells by altering the phosphoinositide-3-kinase, regulatory subunit 3/Akt pathway. Mol Biol Cell 2013;24:42–55.
Wang C, Cao S, Yan Y et al. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer 2010;10:415.
Väisänen MR, Väisänen T, Jukkola-Vuorinen V et al. Expression of toll-like receptor-9 is increased in poorly differentiated prostate tumors. Prostate 2010;70:817–824.
Väisänen MR, Jukkola-Vuorinen A, Vuopala KS, Selander KS, Vaarala MH. Expression of Toll-like receptor-9 is associated with poor progression-free survival in prostate cancer. Oncol Lett 2013;5:1659–1663.
Kauppila JH, Takala H, Selander KS, Lehenkari PP, Saarnio J, Karttunen TJ. Increased Toll-like receptor 9 expression indicates adverse prognosis in oesophageal adenocarcinoma. Histopathology 2011;59:643–649.
Ronkainen H, Hirvikoski P, Kauppila S et al. Absent Toll-like receptor-9 expression predicts poor prognosis in renal cell carcinoma. J Exp Clin Cancer Res 2011;30:84.
Tuomela J, Sandholm J, Karihtala P et al. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res Treat 2012;135:481–493.
Latz E, Schoenemeyer A, Visintin A et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004;5:190–198.
Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localised in the endoplasmic reticulum prior to stimulation. J Immunol 2004;173:1179–1183.
Cui B, Lin H, Yu J, Yu J, Hu Z. Autophagy and the immune response. Adv Exp Med Biol 2019;1206:595–634.
Oh JE, Lee HK. Pattern recognition receptors and autophagy. Front Immunol 2014;5:300.
White E. The role for autophagy in cancer. J Clin Investig 2015;125:42–46.
Muzes G, Constantinovits M, Furi I, Tulassay Z, Sipos F. Interaction of autophagy and toll-like receptors: a regulatory cross-talk—even in cancer cells? Current Drug Targets 2014;15:743–752.
Nitta T, Sato Y, Ren XS et al. Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol 2014;7:4913–4921.
Sasaki M, Nitta T, Sato Y, Nakanuma Y. Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum Pathol 2015;46:202–209.
Mauthe M, Orhon I, Cecilia Rocchi C et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018;14:1435–1455.
Thongchot S, Yongvanit P, Loilome W et al. High expression of HIF-1α, BNIP3 and PI3KC3: hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev 2014;15:5873–5878.
Thongchot S, Loilome W, Yongvanit P et al. Chloroquine exerts anti-metastatic activities under hypoxic conditions in cholangiocarcinoma cells. Asian Pac J Cancer Prev 2015;16:2031–2035.
Jia B, Xue Y, Yan X et al. Autophagy inhibitor chloroquine induces apoptosis of cholangiocarcinoma cells via endoplasmic reticulum stress. Oncol Lett 2018;16:3509–3516.
Fan C, Wang W, Zhao B, Zhang S, Miao J. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg Med Chem 2006;14:3218–3222.
Zheng Y, Zhao YL, Deng X et al. Chloroquine inhibits colon cancer cell growth in vitro and tumorgrowth in vivo via induction of apoptosis. Cancer Investig 2009;27:286–292.
Lin YC. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. J Med Sci 2017;33:215–223.
Shi TT, Yu XX, Yan LJ, **ao HT. Research progress of hydroxychloroquine and autophagy inhibitors on cancer. Cancer Chemother Pharmacol 2017;79:287–294.
Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer 2017;17:528–542.
Chude CI, Amaravadi RK. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci 2017;18:1279.
Marinković M, Šprung M, Buljubašić M, Novak I. Autophagy modulation in cancer: current knowledge on action and therapy. Oxid Med Cell Longev. 2018;2018:8023821.
Ye H, Chen M, Cao F, Huang H, Zhan R, Zheng X. Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol 2016;16:178.
Duffy A, Le J, Sausville E, Emadi A. Autophagy modulation: a target for cancer treatment development. Cancer Chemother Pharmacol. 2015;75:439–447.
Bertin S, Pierrefite-Carle V. Autophagy and toll-like receptors: a new link in cancer cells. Autophagy 2008;4:1086–1089.
Bertin S, Samson M, Pons C et al. Comparative proteomics study reveals that bacterial CpG motifs induce tumor cell autophagy in vitro and in vivo. Mol Cell Proteomics 2008;7:2311–2322.
Lim JS, Kim HS, Nguyen KC, Cho KA. The role of TLR9 in stress-dependent autophagy formation. Biochem Biophys Res Commun 2016;481:219–226.
Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol 2012;30:671–678.
Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer 2018;124:3307–3318.
Zhan L, Li J, Wei B. Autophagy therapeutics: preclinical basis and initial clinical studies. Cancer Chemother Pharmacol 2018;82:923–934.
Bhat P, Kriel J, ShubhaPriya B, Basappa N, Shivananju NS, Loos B. Modulating autophagy in cancer therapy: advancements and challenges for cancer cell death sensitization. Biochem Pharmacol 2018;147:170–182.
Towers CG, Thorburn A. Therapeutic targeting of autophagy. EBioMedicine 2016;14:15–23.
Wang C, Hu Q, Shen HM. Pharmacological inhibitors of autophagy as novel cancer therapeutic agents. Pharmacol Res 2016;105:164–175.
Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene 2016;35:1–11.
Fulda S, Kögel D. Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene. 2015;34:5105–5113.
Zhang Y, Liao Z, Zhang LJ, **ao HT. The utility of chloroquine in cancer therapy. Curr Med Res Opin 2015;31:1009–1013.
Sehgal AR, Konig H, Johnson DE et al. You eat what you are: autophagy inhibition as a therapeutic strategy in leukemia. Leukemia 2015;29:517–525.
Manic G, Obrist F, Kroemer G, Vitale I, Galluzzi L. Chloroquine and hydroxychloroquine for cancer therapy. Mol Cell Oncol 2014;1:e29911.
Reyjal J, Cormier K, Turcotte S. Autophagy and cell death to target cancer cells: exploiting synthetic lethality as cancer therapies. Adv Exp Med Biol 2014;772:167–188.
Cho J, Lee H, Ko H et al. The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma. Oncotarget 2017;8:24932–24948.
Chi H, Li C, Zhao FS et al. Anti-tumor activity of toll-like receptor 7 agonists. Front Pharmacol 2017;8:304.
Li K, Qu S, Chen X, Wu Q, Shi M. Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. Int J Mol Sci 2017;18:404.
Khajeh Alizadeh Attar M, Anwar MA, Eskian M, Keshavarz-Fathi M, Choi S, Rezaei N. Basic understanding and therapeutic approaches to target toll-like receptors in cancerous microenvironment and metastasis. Med Res Rev 2018;38:1469–1484.
Dajon M, Iribarren K, Cremer I. Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology 2017;222:89–100.
Maes H, Kuchnio A, Peric A et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 2014;26:190–206.
Weyerhäuser P, Kantelhardt SR, Kim EL. Re-purposing chloroquine for glioblastoma: potential merits and confounding variables. Front Oncol 2018;8:335.
Acknowledgments
This study was funded by the Egyptian Government (received by FEM) and Liver Failure Group (provided by RJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, F.E., Jalan, R., Minogue, S. et al. Inhibition of TLR7 and TLR9 Reduces Human Cholangiocarcinoma Cell Proliferation and Tumor Development. Dig Dis Sci 67, 1806–1821 (2022). https://doi.org/10.1007/s10620-021-06973-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06973-9