Abstract
In this article, preparation of novel spherical MgCl2 supported α-diimine nickel (II) catalysts for ethylene polymerization in slurry phase is reported. α-Diimine ligands were synthesized by condensation reaction of 2, 6-disubstituted alkyls or aryls anilines and Ace naphthoquinone Which have hydroxyl functionality in their para-position. Hydroxyl functionalized α-diimine attached strongly on to the spherical MgCl2 support surface by dative bonding. No linker was needed to attach the complexes onto the support surface and the amount of loaded Nickel was controllable to improve morphology and especially bulk density of polymer powder. A significant reduction in catalysts activity has happened when homogeneous catalysts were supported onto silica but this reduction was decreased when they were supported onto thermally treated spherical MgCl2. As homogeneous bis(N,N′-(4-(3-hydroxyl-propyl)-2,6-di[(4-tert-butyl-phenyl)-phenyl) amino] Ace naphthoquinone Nickel dibromide(d) showed the highest activity among other evaluated homogeneous catalysts, its MgCl2 supported catalyst (d/S-MgCl2) has shown the highest activity among MgCl2 supported catalysts too. These MgCl2 supported catalysts were pre-polymerized in presence of ethylene monomer in the mild polymerization condition to yield a pre-polymerized catalyst with polymer/catalyst weight ratio equal to six. Ethylene polymerization was carried out to make spherical particles of polyethylene without reactor fouling by these pre-polymerized catalysts. Clearly, it is shown in SEM images that the spherical morphology of MgCl2 support is replicated in the produced polymer. The molecular weight and molecular weight distribution of produced polymer with MgCl2 supported catalysts were higher than those produced by homogeneous catalysts.
Graphic Abstract
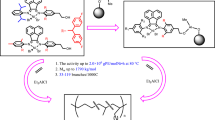
α–Diimine nickel (II) complexes have hydroxy functionality where produce strong dative bonding onto spherical MgCl2. This bonding is strong enough that these catalysts are suitable for slurry polymerization of ethylene without reactor fouling due to catalyst leaching from support. The chemical structure of MgCl2 leads to high active supported catalysts. The molecular weight and polydispersity index of produced polymrers using these supported catalysts are higher than those produced by equivalent homogeneous catalysts and are controllable by selection of appropriate ligand for used α–diimine nickel (II) complex or hydrogen concentration in ethylene polymerization.






Similar content being viewed by others
References
Meinhard D, Wegner M, Kipiani G, Hearley A, Reuter P, Fischer S, Marti O, Rieger B (2007) J Am Chem Soc 129(29):9182–9191
Schmid M, Eberhardt R, Klinga M, Leskela M, Rieger B (2001) Organometallics 20(11):2321–2330
Yiyoung C, Soares JBP (2010) Polymer 51(11):2271–2276
Hlatky GG (2000) Chem Rev 100:1347–1376
Mckenna TF, Soares JBP (2001) Chem Eng Sci 56:3931–3949
Chien JCW (1999) Top Catal 7:23–26
Carnahan EM, Jacobsen GB (2000) Catal Technol 4:74–88
Pflugl PP, Brookhart M (2002) Macromolecules 35:6074–6076
Ye Z, Alsyouri H, Zhu S, Lin YS (2003) Polymer 44:969
Xue X, Yang X, **ao Y, Zhang Q, Wang H (2004) Polymer 45:2877–2882
Mackenzie BP, Moody LS, Killian CN, Lavoie GG (1998) Patent WO 9962968
Vaughan GA, Canich JAM, Matsunaga PT (1996) Patent WO9748736
Simon LC, Patel H, Soares JBP, De Souza RF (2001) Macromol Chem Phys 202:3237–3247
Alobaidi F, Ye Z, Zhu S (2003) Macromol Chem Phys 204:1653
Bennett AM, Moody LS, McLain SD. WO 9856832
Choi Y, Soares JBP (2009) Macromol Chem Phys 210:1979–1988
Schrekker HS, Kotov V, Preishuber-Pflugl P, White P, Brookhart M (2006) Macromolecules 39:6341–6354
Severn JR, Chadwick JC, Van Castelli V (2004) Macromolecules 37:6258–6259
Jiang H, He F, Wang H (2009) J Polym Res 16:183–189
Jiang H, Wu Q, Zhu F, Wang H (2007) J Appl Polym Sci 103:1483–1489
Jiang H, Lu J, Wang F (2010) Polm Bull. https://doi.org/10.1007/s00289-010-0244-7
Canich JAM, Gindelberger DE, Matsunaga PT,Vaughan GA, Squire KR. WO 9748736
Kirsten MC (1999) Top Catal 7:89
Chen Z, Brookhart M (2018) Acc Chem Res 51:1831
Kaiser JM, Long BK (2018) Coord Chem Rev 372:141
Tan C, Chen C (2019) Angew Chem Int Ed 58:7192
Wang F, Chen C (2019) Polym Chem 10:2354
Jalali Dil E, Pourmahdian S, Vatankhah M, Afshar Taromi F (2010) Polym Bull 64:445–457
Monji M, Pourmahdian S, Vatankhah M, Afshar Taromi F (2009) J Appl Polym Sci 112:3663–3668
Kreutzer J (2018) Nat Rev Chem 2:6
Chen C (2018) ACS Catal 8:5506
Vatankhah-Varnoosfaderani, M, Pourmahdian S, Afshar-Taromi F, Amini A, Karimkhani V, Submitted in Macromolecules, ma-2011-011014.
Gibson VC (2006) Science 312:703–704
Zintl M, Rieger B (2007) Angew Chem Int Ed 46:333–335
Karimi B, Enders D (2006) Org Lett 8(6):1237–1240
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kianfar, E., Azimikia, R. & Faghih, S.M. Simple and Strong Dative Attachment of α-Diimine Nickel (II) Catalysts on Supports for Ethylene Polymerization with Controlled Morphology. Catal Lett 150, 2322–2330 (2020). https://doi.org/10.1007/s10562-020-03116-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03116-z