Abstract
Cyclin-dependent kinase 7 (CDK7), along with cyclin H and MAT1, forms the CDK-activating complex (CAK), which directs progression through the cell cycle via T-loop phosphorylation of cell cycle CDKs. CAK is also a component of the general transcription factor, TFIIH. CDK7-mediated phosphorylation of RNA polymerase II (Pol II) at active gene promoters permits transcription. Cell cycle dysregulation is an established hallmark of cancer, and aberrant control of transcriptional processes, through diverse mechanisms, is also common in many cancers. Furthermore, CDK7 levels are elevated in a number of cancer types and are associated with clinical outcomes, suggestive of greater dependence on CDK7 activity, compared with normal tissues. These findings identify CDK7 as a cancer therapeutic target, and several recent publications report selective CDK7 inhibitors (CDK7i) with activity against diverse cancer types. Preclinical studies have shown that CDK7i cause cell cycle arrest, apoptosis and repression of transcription, particularly of super-enhancer-associated genes in cancer, and have demonstrated their potential for overcoming resistance to cancer treatments. Moreover, combinations of CDK7i with other targeted cancer therapies, including BET inhibitors, BCL2 inhibitors and hormone therapies, have shown efficacy in model systems. Four CDK7i, ICEC0942 (CT7001), SY-1365, SY-5609 and LY3405105, have now progressed to Phase I/II clinical trials. Here we describe the work that has led to the development of selective CDK7i, the current status of the most advanced clinical candidates, and discuss their potential importance as cancer therapeutics, both as monotherapies and in combination settings. ClinicalTrials.gov Identifiers: NCT03363893; NCT03134638; NCT04247126; NCT03770494.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
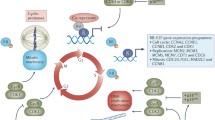
Cyclin-dependent kinase 7 (CDK7), along with cyclin H and MAT1, comprises the CDK-activating kinase (CAK), which provides the T-loop phosphorylation required for activation of CDKs 1,2, 4 and 6, which drive cell cycle progression (Table 1, Fig. 1a) [1,2,3,4]. CAK also has a role in the regulation of transcription, as a component of the general transcription factor TFIIH. At active gene promoters, CDK7 phosphorylates the C-terminal domain (CTD) of RNA polymerase II (Pol II), at serine 5 (Ser5), to facilitate transcription initiation (Table 1, Fig. 1b) [5,6,7]. CDK7 also phosphorylates CDK9, which in turn phosphorylates the Pol II CTD at Ser2, to drive transcription elongation [8]. The activities of a variety of transcription factors, including p53 [9, 10], retinoic acid receptor [11,12,13], oestrogen receptor [14, 15] and androgen receptor [16, 17], are also regulated by CDK7-mediated phosphorylation (Table 1).
Overview of the regulation of CAK and the role of CDK7 in regulating the cell cycle (a) and transcription (b). CAK = CDK activating kinase, CDK = cyclin-dependent kinase, CK2 = protein kinase CK2, G1 = gap phase 1, G2 = gap phase 2, M = mitosis, P = phosphate, PKCι = protein kinase C iota, Pol II = RNA polymerase II, S = synthesis, TFIIH = transcription factor II H
Because of its dual role in regulating the cell cycle and transcription, CDK7 has been studied as an anticancer drug target, and a number of selective inhibitors of CDK7 have been developed and investigated as cancer therapies. Preclinical studies have revealed that cancer cells can be preferentially targeted by transcriptional inhibition, at least in part because they are more reliant than normal cells on high levels of super-enhancer (SE)-driven transcription [18, 19] mediated by specific oncogenic drivers, such as RUNX1 in acute lymphoblastic lymphoma (ALL) [20] and N-MYC in neuroblastoma [21]. To date, four selective CDK7 inhibitors, ICEC0942 [22], SY-1365 [23], SY-5609 [24, 25] and LY340515 [26], have progressed to Phase I/II clinical trial for the treatment of advanced solid malignancies.
In this review we outline the role of CDK7 in both normal and tumour cells and the rationale for inhibiting CDK7 in cancer. We also discuss the development of selective CDK7 inhibitors, their mechanism of action in cancer and their potential for use in combination therapies.
2 CDK7 function
2.1 CAK structure and regulation
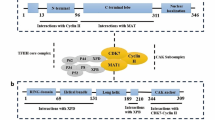
CDK7 is a 346 amino acid kinase, having a predicted molecular mass of 39 kDa, with an N-terminal cyclin H-binding region and a C-terminal MAT1 binding region [27]. A single crystal structure has been reported for CDK7 bound to ATP, in the inactive conformation, the structure being similar to that of the inactive conformation of ATP-bound CDK2 [28]. Cyclin H binding is obligatory for CDK7 kinase activity, whilst the addition of MAT1 stabilises the trimeric CAK complex and anchors it to TFIIH [27]. In addition, cyclin H and MAT1 binding have been shown to regulate CDK7 substrate specificity, with the trimeric CDK7-cyclin H-MAT1 complex having greater kinase activity for Pol II, in comparison to CDK7-cyclin H, which preferentially phosphorylates CDK2 [27,28,29].
The T-loop of CDK7 can be phosphorylated at two positions, threonine 170 (Thr170) and Ser164, enhancing both its kinase activity and ability to bind cyclin H [6]. Furthermore, T-loop phosphorylation of CDK7 seems to direct substrate specificity, with Thr170 phosphorylation stimulating activity towards Pol II over CDK2 [29]. In vitro, CDK1 and CDK2 can phosphorylate CDK7 and as substrates of CDK7 themselves; this hints at the possibility of a reinforcement activation loop between these CDKs [30]. In addition, protein kinase C iota (PKCι), acting downstream of PI3K signalling, can phosphorylate CDK7 at Thr170 (Fig. 1a) [31,32,33,34,35].
Regulation of CAK activity may also be mediated through phosphorylation of cyclin H. CK2 can activate CAK in vitro, via phosphorylation of cyclin H at Thr315 (Fig. 1a) [36], whereas CDK8 has been shown to negatively regulate transcription initiation, via phosphorylation of cyclin H at Ser5 and Ser304 (Fig. 1a) [37]. Furthermore, CDK7 complexed with cyclin H and/or the trimeric CAK can phosphorylate cyclin H in vitro. This autophosphorylation reduces activity of CDK7-cyclin H but has no apparent effect on CDK7-cyclin H-MAT1 activity. This suggests that MAT1 binding aids maintenance of the transcriptional activity of CAK by preventing regulation by cyclin H phosphorylation [28].
An additional means of CDK7 regulation has been observed in mouse neural progenitor cells, where the microRNA (miRNA) miR-210 regulates cell cycle progression by modulating expression levels of CDK7 [38]. This raises the possibility that there may be additional miRNAs that regulate CAK expression and activity in other cellular contexts. There is clearly more to be discovered with regard to the regulation of CDK7 and CAK activity and the identification of players acting upstream of CDK7 could potentially provide additional means by which to manipulate CDK7 activity.
2.2 CDK7 in the cell cycle
CDK7 controls the cell cycle by phosphorylating the cell cycle CDKs 1, 2, 4 and 6 in their T-loops, to promote their activities (Fig. 1a) [1]. Both CDK1 and CDK2 are activated by CDK7-mediated T-loop phosphorylation, at Thr161 and Thr160, respectively (Table 1) [2, 20,21,22, 39]. Inhibiting CDK7 during G1 prevents CDK2 activation and delays S phase, whilst inhibition of CDK7 during S/G2 prevents CDK1 activation and mitotic entry [2, 22]. Whilst CDK7 can phosphorylate CDK2 prior to its binding to cyclin, and is not strictly required for the formation of CDK2-cyclin complexes, CDK7 phosphorylates CDK1 in concert with cyclin B binding and is required for the stabilisation of CDK1-cyclin B complexes [2, 40].
Full commitment to the cell cycle is controlled at the restriction point, through phosphorylation of retinoblastoma (RB) by CDK4/6-cyclin D, in response to mitogens (Fig. 1a). CDK7 phosphorylates both CDK4 and CDK6 in their T-loops, at Thr172 and Thr177 (Table 1), respectively, and CDK7 inhibition prevents their RB kinase activity, halting G1 progression [3, 4]. Although expression levels of the CAK components remain constant throughout the cell cycle, T-loop phosphorylation of CDK7 increases when cells are released from serum starvation [3]. Therefore, a mitogen-induced cascade of CDK T-loop phosphorylation regulates progression through G1 [3].
Unlike cyclin-bound CDK2, which remains phosphorylated for up to 12 hours after CDK7 inhibition, CDK4 and CDK6 activity is rapidly lost following CDK7 inhibition [3]. This difference is likely due to structural differences between the complexes; the T-loop of CDK2 is protected from dephosphorylation by cyclin binding, whereas the T-loops of cyclin D-bound CDK4/6 remain exposed to phosphatases [3]. As a result, CDK7 activity is required to maintain CDK4/6 activity during G1 whilst being required only for initial activation of CDK1 and CDK2 during S/G2 [3].
2.3 CDK7 in transcription
CDK7 regulates gene expression, as a component of the general transcription factor complex, TFIIH (Fig. 1b). TFIIH is composed of two distinct sub-complexes: the core complex, which contains two DNA helicases, xeroderma pigmentosum type B (XPB) and xeroderma pigmentosum type D (XPD), along with five other structural and regulatory proteins, and the CAK complex. CAK is recruited to the core TFIIH complex via a reversible interaction between the ARCH domain of XPD and the latch domain of MAT1 [41, 42]. TFIIH is recruited by TFIIE to active gene promoters, where it joins the other assembled general transcription factors (TFs), and Pol II, in the preinitiation complex (PIC) [27]. The composition of TFIIH and the structure of the PIC have recently been reviewed by Rimel and Taatjes [27].
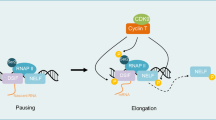
After DNA is unwound at the transcription start site (TSS) by XPB [43], Pol II must be released from the PIC to initiate transcription, in a CDK7-regulated process termed promoter escape [5]. The CTD of mammalian RPB1, the largest subunit of Pol II, contains 52 repeats of a heptad sequence, conforming to the consensus Y1-S2-P3-T4-S5-P6-S7, the residues of which can be sequentially phosphorylated to regulate Pol II activity throughout the transcription cycle [44]. Whilst unphosphorylated, Pol II remains anchored to the PIC, via an interaction with the mediator complex (another PIC component) [5]. CDK7 phosphorylates Ser5 and Ser7 of the Pol II CTD at gene promoters [6, 7]; Ser5 phosphorylation facilitates the release of Pol II from mediator, allowing Pol II to escape the PIC and initiate transcription (Table 1, Fig. 1b) [5, 45]. The precise function of CDK7-directed Ser7 phosphorylation is as yet unclear, but evidence suggests that Ser7 phosphorylation may promote the transcription and post-transcriptional processing of small nuclear RNA transcripts, by facilitating an interaction between the integrator complex and Pol II [46].
After promoter escape, Pol II generally generates a transcript of around 20–80 bases, before halting progress, in a process known as promoter-proximal pausing, which likely functions as a checkpoint to ensure the establishment of a range of co-transcriptional processes [6, 47]. CDK7 is required for the recruitment of two complexes, the DRB sensitivity inducing factor (DSIF) and the negative elongation factor (NELF), both of which are required to establish the promoter-proximal pause [6, 8, 48,49,50]. For the release of paused Pol II and commencement of the productive elongation phase of transcription, the activity of CDK9, as a component of the positive transcription elongation factor (P-TEFb), is required [8]. Like the cell cycle CDKs, for full functionality, CDK9 must undergo T-loop phosphorylation by CDK7 (Table 1, Fig. 1b) [8]. Therefore, CDK7 plays a role in both establishing the promoter-proximal pause and in release from the pause, and inhibition of CDK7 has been shown to increase the amount of Pol II paused at promoter-proximal regions [6, 51]. Active CDK9 phosphorylates the Pol II CTD, on Ser2, promoting transcriptional elongation [52]; therefore, there is an indirect requirement for CDK7 activity after Pol II pause release.
CDK7 also regulates further transcriptional processes; for example, CTD phosphorylation by CDK7 allows the co-transcriptional interaction of Pol II with enzymes that add the 5′-monomethyl-guanosine cap to nascent RNA transcripts [50]. Additionally, CDK7 is necessary for appropriate transcription termination, with read-through transcription observed upon CDK7 inhibition [6]. CDK12 and CDK13 are also involved in regulating transcription by phosphorylating the Pol II CTD during elongation [53]. In vitro, CDK12 can phosphorylate Ser2, Ser5 and Ser7 [54], whereas CDK13 can phosphorylate Ser2 and Ser5 [55]. Like the previously discussed CDKs, T-loop phosphorylation is necessary for CDK12/13 activation and is likely mediated by CDK7 [54, 56]; thus, it is probable that additional transcriptional substrates of CDK7, and further roles in transcriptional regulation, remain to be identified.
Genetic targeting of Mat1 or Cdk7 in mice is early embryonic lethal and cells cultured from embryos of these animals fail to enter S phase [57, 58]. The activities of Cdks 2, 4 and 6 are reduced in mouse embryonic fibroblasts (MEFs) with Cdk7 knockout, indicating that Cdk7 has an essential role in cell proliferation [58]. Cdk7 targeting in adult animals results in phenotypically normal low-proliferating tissues, such as the liver, kidney or cerebellum. However, in rapidly dividing epithelial tissues, Cdk7 expression is retained due to tissue renewal sustained by stem cells with incomplete Cdk7 knockout. This eventually leads to stem cell exhaustion and premature ageing [58]. Interestingly, MEFs lacking Cdk7 expression have unaltered Pol II CTD Ser5 phosphorylation and a largely unchanged gene expression program, indicating that Cdk7 is dispensable for de novo transcription [58]. This raises the possibility that another Pol II CTD kinase can compensate for a lack of Cdk7.
2.4 CDK7 as a regulator of transcription factor activity
Alongside its critical role in directing transcription by Pol II, CDK7 phosphorylates a number of TFs, functioning to either promote their activities and/or regulate their degradation (Table 1). The activity of retinoic acid receptor ⍺ (RAR⍺) is promoted by XPD-dependent phosphorylation of Ser77 by CDK7 [11, 13]. Likewise, the activity of RARγ is also modulated by phosphorylation by TFIIH-incorporated CDK7 [12]. CDK7, as part of TFIIH, mediates ligand-dependent phosphorylation of oestrogen receptor ⍺ (ER⍺) at Ser118 [14, 15], regulating the activity and turnover of the TF [59, 60]. Phosphorylation by CDK7, at Ser515 in the transcription activation function of androgen receptor (AR), has also been reported [16, 17]. Additionally, CDK7 can phosphorylate p53 in a MAT1-dependent fashion, at both the C-terminus (between residues 311 and 393) [10] and the N-terminus, at Ser33 [9], the former of which has been shown to stimulate p53 binding to DNA. Evidence that CDK7 phosphorylates Ets1 [61], peroxisome proliferator-activated receptors (PPARs) [62] and E2F1 has also been demonstrated, the latter functioning to trigger E2F1 degradation [63] (Table 1). Recently, the stabilisation of the transcriptional regulators YAP/TAZ was shown to be mediated by CDK7, with phosphorylation of YAP at Ser128 and TAZ at Ser90, preventing their ubiquitination and degradation [64]. At present we have an incomplete understanding of the role CDK7 plays in regulating the activities of sequence-specific transcriptional regulators. Further knowledge in this area may be helpful in informing the use of CDK7 inhibitors in specific cellular contexts.
2.5 CDK7 in DNA repair
TFIIH plays a key role in the nucleotide excision repair (NER) pathway [27], which repairs single-stranded DNA damage, particularly that caused by ultraviolet light. TFIIH is recruited to damaged DNA, where the NER protein, xeroderma pigmentosum group A (XPA), catalyses the release of CAK from the core TFIIH complex, allowing NER to proceed [65]. After DNA repair, CAK reassociates with TFIIH, and the complex resumes its role in transcription [65]. Inhibition of CDK7 kinase activity improves NER efficiency, suggesting that CDK7 negatively regulates NER, directly or indirectly, via phosphorylation of an as yet unidentified substrate(s) [66].
3 CDK7 in cancer
3.1 CDK7 expression in tumours
Two decades ago, immunohistochemical analyses on a range of tumour types indicated that CDK7 expression is elevated in tumour cells compared with their normal counterparts [67]. Since then, numerous studies have provided support for this finding [68,50, 51]; however, it was recently shown that its anti-transcriptional and antitumour activities are reliant on inhibition of CDK12 and CDK13, in addition to CDK7 [130]. Consequently, YKL-5-124 was developed, with a strategy that combined the covalent warhead of THZ1 with the pyrrolidinopyrazole core of the PAK4 inhibitor, PF-3758309 [130]. Like THZ1, YKL-5-124 covalently links to Cys312 of CDK7 but does not affect the activities of CDK12 and 13 (Fig. 3b, Table 3) [130]. An analogue of THZ1, with altered regiochemistry of the acrylamide and increased in vivo stability has also been developed and was designated THZ2 (Fig. 3b, Table 3) [ CDK7 has a dual role in driving the cell cycle and transcription, is upregulated in a variety of cancers and has emerged as a promising cancer therapeutic target. At least ten selective inhibitors of CDK7, with activity against a wide range of cancer types, have been developed, their antitumour action likely mediated both through cell cycle arrest and inhibition of oncogenic transcriptional programs. In the preclinical setting, these inhibitors have demonstrated potential to overcome treatment-resistant cancer, both as monotherapies, and in combination with other cancer drugs. To date, four CDK7 inhibitors have progressed to Phase I/II clinical trial for the treatment of advanced solid malignancies. Whilst ABC-transporters can mediate resistance to some CDK7 inhibitors, additional factors that influence tumour response to CDK7 inhibition are yet to be identified. Further efforts to elucidate mechanisms of response, and to define patient selection strategies, will help to facilitate the clinical utility of CDK7 inhibitors.6 Conclusions
References
Schachter, M. M., & Fisher, R. P. (2013). The CDK-activating kinase Cdk7. Cell Cycle, 12(20), 3239–3240. https://doi.org/10.4161/cc.26355.
Larochelle, S., Merrick, K. A., Terret, M.-E., Wohlbold, L., Barboza, N. M., Zhang, C., Shokat, K. M., Jallepalli, P. V., & Fisher, R. P. (2007). Requirements for Cdk7 in the assembly of Cdk1/Cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Molecular Cell, 25(6), 839–850. https://doi.org/10.1016/j.molcel.2007.02.003.
Schachter, M. M., Merrick, K. A., Larochelle, S., Hirschi, A., Zhang, C., Shokat, K. M., Rubin, S. M., & Fisher, R. P. (2013). A Cdk7-Cdk4 T-loop phosphorylation Cascade promotes G1 progression. Molecular Cell, 50(2), 250–260. https://doi.org/10.1016/j.molcel.2013.04.003.
Bisteau, X., Paternot, S., Colleoni, B., Ecker, K., Coulonval, K., De Groote, P., et al. (2013). CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genetics, 9(5), e1003546. https://doi.org/10.1371/journal.pgen.1003546.
Wong, K. H., **, Y., & Struhl, K. (2014). TFIIH phosphorylation of the pol II CTD stimulates mediator dissociation from the Preinitiation complex and promoter escape. Molecular Cell, 54(4), 601–612. https://doi.org/10.1016/j.molcel.2014.03.024.
Glover-Cutter, K., Larochelle, S., Erickson, B., Zhang, C., Shokat, K., Fisher, R. P., et al. (2009). TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Molecular and Cellular Biology, 29(20), 5455–5464. https://doi.org/10.1128/mcb.00637-09.
Akhtar, M. S., Heidemann, M., Tietjen, J. R., Zhang, D. W., Chapman, R. D., Eick, D., & Ansari, A. Z. (2009). TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Molecular Cell, 34(3), 387–393. https://doi.org/10.1016/j.molcel.2009.04.016.
Larochelle, S., Amat, R., Glover-Cutter, K., Sansó, M., Zhang, C., Allen, J. J., et al. (2012). Cyclin-dependent kinase control of the initiation-to-elongation switch of RNA polymerase II. Nature Structural & Molecular Biology, 19, 1108, https://doi.org/10.1038/nsmb.2399https://www.nature.com/articles/nsmb.2399#supplementary-information.
Ko, L. J., Shieh, S. Y., Chen, X., Jayaraman, L., Tamai, K., Taya, Y., Prives, C., & Pan, Z. Q. (1997). p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Molecular and Cellular Biology, 17(12), 7220–7229. https://doi.org/10.1128/mcb.17.12.7220.
Lu, H., Fisher, R. P., Bailey, P., & Levine, A. J. (1997). The CDK7-cycH-p36 complex of transcription factor IIH phosphorylates p53, enhancing its sequence-specific DNA binding activity in vitro. Molecular and Cellular Biology, 17(10), 5923–5934. https://doi.org/10.1128/mcb.17.10.5923.
Keriel, A., Stary, A., Sarasin, A., Rochette-Egly, C., & Egly, J.-M. (2002). XPD mutations prevent TFIIH-dependent transactivation by nuclear receptors and phosphorylation of RARα. Cell, 109(1), 125–135. https://doi.org/10.1016/S0092-8674(02)00692-X.
Bastien, J., Adam-Stitah, S., Riedl, T., Egly, J.-M., Chambon, P., & Rochette-Egly, C. (2000). TFIIH interacts with the retinoic acid receptor γ and phosphorylates its AF-1-activating domain through cdk7. Journal of Biological Chemistry, 275(29), 21896–21904. https://doi.org/10.1074/jbc.M001985200.
Rochette-Egly, C., Adam, S., Rossignol, M., Egly, J.-M., & Chambon, P. (1997). Stimulation of RARα activation function AF-1 through binding to the general transcription factor TFIIH and phosphorylation by CDK7. Cell, 90(1), 97–107. https://doi.org/10.1016/S0092-8674(00)80317-7.
Chen, D., Riedl, T., Washbrook, E., Pace, P. E., Coombes, R. C., Egly, J.-M., & Ali, S. (2000). Activation of estrogen receptor α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Molecular Cell, 6(1), 127–137. https://doi.org/10.1016/S1097-2765(05)00004-3.
Chen, D., Washbrook, E., Sarwar, N., Bates, G. J., Pace, P. E., Thirunuvakkarasu, V., Taylor, J., Epstein, R. J., Fuller-Pace, F. V., Egly, J. M., Coombes, R. C., & Ali, S. (2002). Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene, 21(32), 4921–4931. https://doi.org/10.1038/sj.onc.1205420.
Lee, D. K., Duan, H. O., & Chang, C. (2000). From Androgen receptor to the general transcription factor TFIIH: identification of cdk activating kinase (CAK) as an androgen receptor NH2- terminal associated coactivator. Journal of Biological Chemistry, 275(13), 9308–9313. https://doi.org/10.1074/jbc.275.13.9308.
Chymkowitch, P., Le May, N., Charneau, P., Compe, E., & Egly, J.-M. (2011). The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. The EMBO Journal, 30(3), 468–479. https://doi.org/10.1038/emboj.2010.337.
Galbraith, M. D., Bender, H., & Espinosa, J. M. (2019). Therapeutic targeting of transcriptional cyclin-dependent kinases. Transcription, 10(2), 118–136. https://doi.org/10.1080/21541264.2018.1539615.
Bradner, J. E., Hnisz, D., & Young, R. A. (2017). Transcriptional addiction in cancer. Cell, 168(4), 629–643. https://doi.org/10.1016/j.cell.2016.12.013.
Kwiatkowski, N., Zhang, T., Rahl, P. B., Abraham, B. J., Reddy, J., Ficarro, S. B., Dastur, A., Amzallag, A., Ramaswamy, S., Tesar, B., Jenkins, C. E., Hannett, N. M., McMillin, D., Sanda, T., Sim, T., Kim, N. D., Look, T., Mitsiades, C. S., Weng, A. P., Brown, J. R., Benes, C. H., Marto, J. A., Young, R. A., & Gray, N. S. (2014). Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature, 511(7511), 616–620. https://doi.org/10.1038/nature13393.
Chipumuro, E., Marco, E., Christensen, C. L., Kwiatkowski, N., Zhang, T., Hatheway, C. M., Abraham, B. J., Sharma, B., Yeung, C., Altabef, A., Perez-Atayde, A., Wong, K. K., Yuan, G. C., Gray, N. S., Young, R. A., & George, R. E. (2014). CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell, 159(5), 1126–1139. https://doi.org/10.1016/j.cell.2014.10.024.
Patel, H., Periyasamy, M., Sava, G. P., Bondke, A., Slafer, B. W., Kroll, S. H. B., Barbazanges, M., Starkey, R., Ottaviani, S., Harrod, A., Aboagye, E. O., Buluwela, L., Fuchter, M. J., Barrett, A. G. M., Coombes, R. C., & Ali, S. (2018). ICEC0942, an orally bioavailable selective inhibitor of CDK7 for cancer treatment. Molecular Cancer Therapeutics, 17(6), 1156–1166. https://doi.org/10.1158/1535-7163.Mct-16-0847.
Hu, S., Marineau, J. J., Rajagopal, N., Hamman, K. B., Choi, Y. J., Schmidt, D. R., et al. (2019). Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Research, canres.0119.2019, https://doi.org/10.1158/0008-5472.Can-19-0119.
Hu, S., Marineau, J., Hamman, K., Bradley, M., Savinainen, A., Alnemy, S., et al. (2019). Abstract 4421: SY-5609, an orally available selective CDK7 inhibitor demonstrates broad anti-tumor activity in vivo. Cancer Research, 79(13 Supplement), 4421–4421. https://doi.org/10.1158/1538-7445.Am2019-4421.
Johannessen, L. H., Hu, S., Ke, N., D'Ippolito, A., Rajagopal, N., Marineau, J., et al. (2019). Abstract C091: Preclinical evaluation of PK, PD, and antitumor activity of the oral, non-covalent, potent and highly selective CDK7 inhibitor, SY-5609, provides rationale for clinical development in multiple solid tumor indications. Molecular Cancer Therapeutics, 18(12 Supplement), C091–C091. https://doi.org/10.1158/1535-7163.Targ-19-c091.
Coates, D. A., Montero, C., Patel, B. K. R., Remick, D. M., & Yadav, V. (2019). Compounds useful for inhibiting CDK7. United States: Eli Lilly and Company. IN, US: Indianapolis.
Rimel, J. K., & Taatjes, D. J. (2018). The essential and multifunctional TFIIH complex. Protein Science, 27(6), 1018–1037. https://doi.org/10.1002/pro.3424.
Lolli, G., Lowe, E. D., Brown, N. R., & Johnson, L. N. (2004). The crystal structure of human CDK7 and its protein recognition properties. Structure, 12(11), 2067–2079. https://doi.org/10.1016/j.str.2004.08.013.
Larochelle, S., Chen, J., Knights, R., Pandur, J., Morcillo, P., Erdjument-Bromage, H., Tempst, P., Suter, B., & Fisher, R. P. (2001). T-loop phosphorylation stabilizes the CDK7-cyclin H-MAT1 complex in vivo and regulates its CTD kinase activity. The EMBO Journal, 20(14), 3749–3759. https://doi.org/10.1093/emboj/20.14.3749.
Garrett, S., Barton, W. A., Knights, R., **, P., Morgan, D. O., & Fisher, R. P. (2001). Reciprocal activation by Cyclin-dependent kinases 2 and 7 is directed by substrate specificity determinants outside the T loop. Molecular and Cellular Biology, 21(1), 88–99. https://doi.org/10.1128/mcb.21.1.88-99.2001.
Desai, S. R., Pillai, P. P., Patel, R. S., McCray, A. N., Win-Piazza, H. Y., & Acevedo-Duncan, M. E. (2011). Regulation of Cdk7 activity through a phosphatidylinositol (3)-kinase/PKC-ι-mediated signaling cascade in glioblastoma. Carcinogenesis, 33(1), 10–19. https://doi.org/10.1093/carcin/bgr231.
Pillai, P., Desai, S., Patel, R., Sajan, M., Farese, R., Ostrov, D., & Acevedo-Duncan, M. (2011). A novel PKC-ι inhibitor abrogates cell proliferation and induces apoptosis in neuroblastoma. The International Journal of Biochemistry & Cell Biology, 43(5), 784–794. https://doi.org/10.1016/j.biocel.2011.02.002.
Acevedo-Duncan, M., Patel, R., Whelan, S., & Bicaku, E. (2002). Human glioma PKC-ι and PKC-βII phosphorylate cyclin-dependent kinase activating kinase during the cell cycle. Cell Proliferation, 35(1), 23–36. https://doi.org/10.1046/j.1365-2184.2002.00220.x.
Ni, S., Chen, L., Li, M., Zhao, W., Shan, X., Wu, M., Cheng, J., Liang, L., Wang, Y., Jiang, W., Zhang, J., & Ni, R. (2016). PKC iota promotes cellular proliferation by accelerated G1/S transition via interaction with CDK7 in esophageal squamous cell carcinoma. Tumor Biology, 37(10), 13799–13809. https://doi.org/10.1007/s13277-016-5193-9.
Ghezzi, C., Wong, A., Chen, B. Y., Ribalet, B., Damoiseaux, R., & Clark, P. M. (2019). A high-throughput screen identifies that CDK7 activates glucose consumption in lung cancer cells. Nature Communications, 10(1), 5444. https://doi.org/10.1038/s41467-019-13334-8.
Schneider, E., Kartarius, S., Schuster, N., & Montenarh, M. (2002). The cyclin H/cdk7/Mat1 kinase activity is regulated by CK2 phosphorylation of cyclin H. Oncogene, 21(33), 5031–5037. https://doi.org/10.1038/sj.onc.1205690.
Akoulitchev, S., Chuikov, S., & Reinberg, D. (2000). TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature, 407(6800), 102–106. https://doi.org/10.1038/35024111.
Abdullah, A. I., Zhang, H., Nie, Y., Tang, W., & Sun, T. (2016). CDK7 and miR-210 co-regulate cell-cycle progression of neural progenitors in the develo** Neocortex. Stem Cell Reports, 7(1), 69–79. https://doi.org/10.1016/j.stemcr.2016.06.005.
Ali, S., Heathcote, D. A., Kroll, S. H. B., Jogalekar, A. S., Scheiper, B., Patel, H., Brackow, J., Siwicka, A., Fuchter, M. J., Periyasamy, M., Tolhurst, R. S., Kanneganti, S. K., Snyder, J. P., Liotta, D. C., Aboagye, E. O., Barrett, A. G. M., & Coombes, R. C. (2009). The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Research, 69(15), 6208–6215. https://doi.org/10.1158/0008-5472.Can-09-0301.
Merrick, K. A., Larochelle, S., Zhang, C., Allen, J. J., Shokat, K. M., & Fisher, R. P. (2008). Distinct activation pathways confer cyclin-binding specificity on Cdk1 and Cdk2 in human cells. Molecular Cell, 32(5), 662–672. https://doi.org/10.1016/j.molcel.2008.10.022.
Abdulrahman, W., Iltis, I., Radu, L., Braun, C., Maglott-Roth, A., Giraudon, C., Egly, J. M., & Poterszman, A. (2013). ARCH domain of XPD, an anchoring platform for CAK that conditions TFIIH DNA repair and transcription activities. Proceedings of the National Academy of Sciences, 110(8), E633–E642. https://doi.org/10.1073/pnas.1213981110.
Luo, J., Cimermancic, P., Viswanath, S., Ebmeier, C. C., Kim, B., Dehecq, M., Raman, V., Greenberg, C. H., Pellarin, R., Sali, A., Taatjes, D. J., Hahn, S., & Ranish, J. (2015). Architecture of the human and yeast general transcription and DNA repair factor TFIIH. Molecular Cell, 59(5), 794–806. https://doi.org/10.1016/j.molcel.2015.07.016.
Tirode, F., Busso, D., Coin, F., & Egly, J.-M. (1999). Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7. Molecular Cell, 3(1), 87–95. https://doi.org/10.1016/S1097-2765(00)80177-X.
Bataille, A. R., Jeronimo, C., Jacques, P.-É., Laramée, L., Fortin, M.-È., Forest, A., Bergeron, M., Hanes, S. D., & Robert, F. (2012). A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Molecular Cell, 45(2), 158–170. https://doi.org/10.1016/j.molcel.2011.11.024.
Jeronimo, C., & Robert, F. (2014). Kin28 regulates the transient association of mediator with core promoters. Nature Structural & Molecular Biology, 21(5), 449–455. https://doi.org/10.1038/nsmb.2810.
Egloff, S., O'Reilly, D., Chapman, R. D., Taylor, A., Tanzhaus, K., Pitts, L., Eick, D., & Murphy, S. (2007). Serine-7 of the RNA polymerase II CTD is specifically required for snRNA gene expression. Science, 318(5857), 1777–1779. https://doi.org/10.1126/science.1145989.
Glover-Cutter, K., Kim, S., Espinosa, J., & Bentley, D. L. (2007). RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nature Structural & Molecular Biology, 15, 71. https://doi.org/10.1038/nsmb1352https://www.nature.com/articles/nsmb1352#supplementary-information.
Kwak, H., & Lis, J. T. (2013). Control of transcriptional elongation. Annual Review of Genetics, 47, 483–508. https://doi.org/10.1146/annurev-genet-110711-155440.
Yamaguchi, Y., Takagi, T., Wada, T., Yano, K., Furuya, A., Sugimoto, S., Hasegawa, J., & Handa, H. (1999). NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell, 97(1), 41–51. https://doi.org/10.1016/S0092-8674(00)80713-8.
Nilson, K. A., Guo, J., Turek, M. E., Brogie, J. E., Delaney, E., Luse, D. S., & Price, D. H. (2015). THZ1 reveals roles for Cdk7 in co-transcriptional cap** and pausing. Molecular Cell, 59(4), 576–587. https://doi.org/10.1016/j.molcel.2015.06.032.
Ebmeier, C. C., Erickson, B., Allen, B. L., Allen, M. A., Kim, H., Fong, N., Jacobsen, J. R., Liang, K., Shilatifard, A., Dowell, R. D., Old, W. M., Bentley, D. L., & Taatjes, D. J. (2017). Human TFIIH kinase CDK7 regulates transcription-associated chromatin modifications. Cell Reports, 20(5), 1173–1186. https://doi.org/10.1016/j.celrep.2017.07.021.
Bacon, C. W., & D’Orso, I. (2019). CDK9: A signaling hub for transcriptional control. Transcription, 10(2), 57–75. https://doi.org/10.1080/21541264.2018.1523668.
Bartkowiak, B., Liu, P., Phatnani, H. P., Fuda, N. J., Cooper, J. J., Price, D. H., Adelman, K., Lis, J. T., & Greenleaf, A. L. (2010). CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes & Development, 24(20), 2303–2316. https://doi.org/10.1101/gad.1968210.
Bösken, C. A., Farnung, L., Hintermair, C., Merzel Schachter, M., Vogel-Bachmayr, K., Blazek, D., et al. (2014). The structure and substrate specificity of human Cdk12/Cyclin K. Nature Communications, 5, 3505, https://doi.org/10.1038/ncomms4505https://www.nature.com/articles/ncomms4505#supplementary-information.
Liang, K., Gao, X., Gilmore, J. M., Florens, L., Washburn, M. P., Smith, E., & Shilatifard, A. (2015). Characterization of human cyclin-dependent kinase 12 (CDK12) and CDK13 complexes in C-terminal domain phosphorylation, gene transcription, and RNA processing. Molecular and Cellular Biology, 35(6), 928–938. https://doi.org/10.1128/mcb.01426-14.
Greifenberg, A. K., Hönig, D., Pilarova, K., Düster, R., Bartholomeeusen, K., Bösken, C. A., Anand, K., Blazek, D., & Geyer, M. (2016). Structural and functional analysis of the Cdk13/Cyclin K complex. Cell Reports, 14(2), 320–331. https://doi.org/10.1016/j.celrep.2015.12.025.
Rossi, D. J., Londesborough, A., Korsisaari, N., Pihlak, A., Lehtonen, E., Henkemeyer, M., & Mäkelä, T. P. (2001). Inability to enter S phase and defective RNA polymerase II CTD phosphorylation in mice lacking Mat1. The EMBO Journal, 20(11), 2844–2856. https://doi.org/10.1093/emboj/20.11.2844.
Ganuza, M., Sáiz-Ladera, C., Cañamero, M., Gómez, G., Schneider, R., Blasco, M. A., Pisano, D., Paramio, J. M., Santamaría, D., & Barbacid, M. (2012). Genetic inactivation of Cdk7 leads to cell cycle arrest and induces premature aging due to adult stem cell exhaustion. The EMBO Journal, 31(11), 2498–2510. https://doi.org/10.1038/emboj.2012.94.
Ali, S., Metzger, D., Bornert, J. M., & Chambon, P. (1993). Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. The EMBO Journal, 12(3), 1153–1160. https://doi.org/10.1002/j.1460-2075.1993.tb05756.x.
Valley, C. C., Métivier, R., Solodin, N. M., Fowler, A. M., Mashek, M. T., Hill, L., et al. (2005). Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor α N terminus. Molecular and Cellular Biology, 25(13), 5417–5428. https://doi.org/10.1128/mcb.25.13.5417-5428.2005.
Drané, P., Compe, E., Catez, P., Chymkowitch, P., & Egly, J.-M. (2004). Selective regulation of vitamin D receptor-responsive genes by TFIIH. Molecular Cell, 16(2), 187–197. https://doi.org/10.1016/j.molcel.2004.10.007.
Compe, E., Drané, P., Laurent, C., Diderich, K., Braun, C., Hoeijmakers, J. H. J., et al. (2005). Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Molecular and Cellular Biology, 25(14), 6065–6076. https://doi.org/10.1128/mcb.25.14.6065-6076.2005.
Vandel, L., & Kouzarides, T. (1999). Residues phosphorylated by TFIIH are required for E2F-1 degradation during S-phase. The EMBO Journal, 18(15), 4280–4291. https://doi.org/10.1093/emboj/18.15.4280.
Cho, Y. S., Li, S., Wang, X., Zhu, J., Zhuo, S., Han, Y., Yue, T., Yang, Y., & Jiang, J. (2019). CDK7 regulates organ size and tumor growth by safeguarding the hippo pathway effector Yki/yap/Taz in the nucleus. Genes & Development, 34, 53–71. https://doi.org/10.1101/gad.333146.119.
Coin, F., Oksenych, V., Mocquet, V., Groh, S., Blattner, C., & Egly, J. M. (2008). Nucleotide excision repair driven by the dissociation of CAK from TFIIH. Molecular Cell, 31(1), 9–20. https://doi.org/10.1016/j.molcel.2008.04.024.
Araújo, S. J., Tirode, F., Coin, F., Pospiech, H., Syväoja, J. E., Stucki, M., Hübscher, U., Egly, J. M., & Wood, R. D. (2000). Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes & Development, 14(3), 349–359. https://doi.org/10.1101/gad.14.3.349.
Bartkova, J., Zemanova, M., & Bartek, J. (1996). Expression of CDK7/CAK in normal and tumour cells of diverse histogenesis, cell-cycle position and differentiation. International Journal of Cancer, 66(6), 732–737. https://doi.org/10.1002/(sici)1097-0215(19960611)66:6<732::Aid-ijc4>3.0.Co;2-0.
Patel, H., Abduljabbar, R., Lai, C.-F., Periyasamy, M., Harrod, A., Gemma, C., Steel, J. H., Patel, N., Busonero, C., Jerjees, D., Remenyi, J., Smith, S., Gomm, J. J., Magnani, L., Gyorffy, B., Jones, L. J., Fuller-Pace, F., Shousha, S., Buluwela, L., Rakha, E. A., Ellis, I. O., Coombes, R. C., & Ali, S. (2016). Expression of CDK7, cyclin H, and MAT1 is elevated in breast cancer and is prognostic in estrogen receptor–positive breast cancer. Clinical Cancer Research, 22(23), 5929–5938. https://doi.org/10.1158/1078-0432.Ccr-15-1104.
Wang, Q., Li, M., Zhang, X., Huang, H., Huang, J., Ke, J., Ding, H., **ao, J., Shan, X., Liu, Q., Bao, B., & Yang, L. (2016). Upregulation of CDK7 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Experimental and Molecular Pathology, 100(3), 514–521. https://doi.org/10.1016/j.yexmp.2016.05.001.
Naseh, G., Mohammadifard, M., & Mohammadifard, M. (2016). Upregulation of cyclin-dependent kinase 7 and matrix metalloproteinase-14 expression contribute to metastatic properties of gastric cancer. IUBMB Life, 68(10), 799–805. https://doi.org/10.1002/iub.1543.
Jiang, L., Huang, R., Wu, Y., Diao, P., Zhang, W., Li, J., Li, Z., Wang, Y., Cheng, J., & Yang, J. (2019). Overexpression of CDK7 is associated with unfavourable prognosis in oral squamous cell carcinoma. Pathology, 51(1), 74–80. https://doi.org/10.1016/j.pathol.2018.10.004.
Tsang, F. H.-C., Law, C.-T., Tang, T.-C. C., Cheng, C. L.-H., Chin, D. W.-C., Tam, W.-S. V., Wei, L., Wong, C. C. L., Ng, I. O. L., & Wong, C. M. (2019). Aberrant super-enhancer landscape in human hepatocellular carcinoma. Hepatology, 69(6), 2502–2517. https://doi.org/10.1002/hep.30544.
Meng, W., Wang, J., Wang, B., Liu, F., Li, M., Zhao, Y., Zhang, C., Li, Q., Chen, J., Zhang, L., Tang, Y., & Ma, J. (2018). CDK7 inhibition is a novel therapeutic strategy against GBM both in vitro and in vivo. Cancer Management and Research, 10, 5747–5758. https://doi.org/10.2147/CMAR.S183696.
Li, B., Ni Chonghaile, T., Fan, Y., Madden, S. F., Klinger, R., O'Connor, A. E., Walsh, L., O'Hurley, G., Mallya Udupi, G., Joseph, J., Tarrant, F., Conroy, E., Gaber, A., Chin, S. F., Bardwell, H. A., Provenzano, E., Crown, J., Dubois, T., Linn, S., Jirstrom, K., Caldas, C., O'Connor, D. P., & Gallagher, W. M. (2017). Therapeutic rationale to target highly expressed CDK7 conferring poor outcomes in triple-negative breast cancer. Cancer Research, 77(14), 3834–3845. https://doi.org/10.1158/0008-5472.Can-16-2546.
Zhang, Z., Peng, H., Wang, X., Yin, X., Ma, P., **g, Y., Cai, M. C., Liu, J., Zhang, M., Zhang, S., Shi, K., Gao, W. Q., di, W., & Zhuang, G. (2017). Preclinical efficacy and molecular mechanism of targeting CDK7-dependent transcriptional addiction in ovarian cancer. Molecular Cancer Therapeutics, 16(9), 1739–1750. https://doi.org/10.1158/1535-7163.Mct-17-0078.
Pavey, S., Johansson, P., Packer, L., Taylor, J., Stark, M., Pollock, P. M., Walker, G. J., Boyle, G. M., Harper, U., Cozzi, S. J., Hansen, K., Yudt, L., Schmidt, C., Hersey, P., Ellem, K. A. O., O'Rourke, M. G. E., Parsons, P. G., Meltzer, P., Ringnér, M., & Hayward, N. K. (2004). Microarray expression profiling in melanoma reveals a BRAF mutation signature. Oncogene, 23(23), 4060–4067. https://doi.org/10.1038/sj.onc.1207563.
Johansson, P., Pavey, S., & Hayward, N. (2007). Confirmation of a BRAF mutation-associated gene expression signature in melanoma. Pigment Cell Research, 20(3), 216–221. https://doi.org/10.1111/j.1600-0749.2007.00375.x.
Kandoth, C., McLellan, M. D., Vandin, F., Ye, K., Niu, B., Lu, C., **e, M., Zhang, Q., McMichael, J. F., Wyczalkowski, M. A., Leiserson, M. D. M., Miller, C. A., Welch, J. S., Walter, M. J., Wendl, M. C., Ley, T. J., Wilson, R. K., Raphael, B. J., & Ding, L. (2013). Mutational landscape and significance across 12 major cancer types. Nature, 502(7471), 333–339. https://doi.org/10.1038/nature12634.
Lawrence, M. S., Stojanov, P., Mermel, C. H., Robinson, J. T., Garraway, L. A., Golub, T. R., Meyerson, M., Gabriel, S. B., Lander, E. S., & Getz, G. (2014). Discovery and saturation analysis of cancer genes across 21 tumour types. Nature, 505(7484), 495–501. https://doi.org/10.1038/nature12912.
Lane, D., & Levine, A. (2010). p53 research: the past thirty years and the next thirty years. Cold Spring Harbor Perspectives in Biology, 2(12). https://doi.org/10.1101/cshperspect.a000893.
Beroukhim, R., Mermel, C. H., Porter, D., Wei, G., Raychaudhuri, S., Donovan, J., Barretina, J., Boehm, J. S., Dobson, J., Urashima, M., Mc Henry, K. T., Pinchback, R. M., Ligon, A. H., Cho, Y. J., Haery, L., Greulich, H., Reich, M., Winckler, W., Lawrence, M. S., Weir, B. A., Tanaka, K. E., Chiang, D. Y., Bass, A. J., Loo, A., Hoffman, C., Prensner, J., Liefeld, T., Gao, Q., Yecies, D., Signoretti, S., Maher, E., Kaye, F. J., Sasaki, H., Tepper, J. E., Fletcher, J. A., Tabernero, J., Baselga, J., Tsao, M. S., Demichelis, F., Rubin, M. A., Janne, P. A., Daly, M. J., Nucera, C., Levine, R. L., Ebert, B. L., Gabriel, S., Rustgi, A. K., Antonescu, C. R., Ladanyi, M., Letai, A., Garraway, L. A., Loda, M., Beer, D. G., True, L. D., Okamoto, A., Pomeroy, S. L., Singer, S., Golub, T. R., Lander, E. S., Getz, G., Sellers, W. R., & Meyerson, M. (2010). The landscape of somatic copy-number alteration across human cancers. Nature, 463(7283), 899–905. https://doi.org/10.1038/nature08822.
Clemons, M., Danson, S., & Howell, A. (2002). Tamoxifen (‘Nolvadex’): a review: Antitumour treatment. Cancer Treatment Reviews, 28(4), 165–180. https://doi.org/10.1016/S0305-7372(02)00036-1.
Nathan, M. R., & Schmid, P. (2017). A review of fulvestrant in breast cancer. Oncology and Therapy, 5(1), 17–29. https://doi.org/10.1007/s40487-017-0046-2.
Hnisz, D., Abraham, B. J., Lee, T. I., Lau, A., Saint-André, V., Sigova, A. A., Hoke, H. A., & Young, R. A. (2013). Super-enhancers in the control of cell identity and disease. Cell, 155(4), 934–947. https://doi.org/10.1016/j.cell.2013.09.053.
Whittaker, S. R., Mallinger, A., Workman, P., & Clarke, P. A. (2017). Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacology & Therapeutics, 173, 83–105. https://doi.org/10.1016/j.pharmthera.2017.02.008.
Conroy, A., Stockett, D. E., Walker, D., Arkin, M. R., Hoch, U., Fox, J. A., & Hawtin, R. E. (2009). SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemotherapy and Pharmacology, 64(4), 723–732. https://doi.org/10.1007/s00280-008-0921-5.
Kaur, G., Stetler-Stevenson, M., Sebers, S., Worland, P., Sedlacek, H., Myers, C., Czech, J., Naik, R., & Sausville, E. (1992). Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. JNCI: Journal of the National Cancer Institute, 84(22), 1736–1740. https://doi.org/10.1093/jnci/84.22.1736.
Losiewicz, M. D., Carlson, B. A., Kaur, G., Sausville, E. A., & Worland, P. J. (1994). Potent inhibition of Cdc2 kinase activity by the flavonoid L86-8275. Biochemical and Biophysical Research Communications, 201(2), 589–595. https://doi.org/10.1006/bbrc.1994.1742.
Carlson, B. A., Dubay, M. M., Sausville, E. A., Brizuela, L., & Worland, P. J. (1996). Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Research, 56(13), 2973–2978.
Chen, R., Keating, M. J., Gandhi, V., & Plunkett, W. (2005). Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood, 106(7), 2513–2519. https://doi.org/10.1182/blood-2005-04-1678.
Asghar, U., Witkiewicz, A. K., Turner, N. C., & Knudsen, E. S. (2015). The history and future of targeting cyclin-dependent kinases in cancer therapy. [review article]. Nature Reviews Drug Discovery, 14, 130. https://doi.org/10.1038/nrd4504https://www.nature.com/articles/nrd4504#supplementary-information.
Byrd, J. C., Lin, T. S., Dalton, J. T., Wu, D., Phelps, M. A., Fischer, B., Moran, M., Blum, K. A., Rovin, B., Brooker-McEldowney, M., Broering, S., Schaaf, L. J., Johnson, A. J., Lucas, D. M., Heerema, N. A., Lozanski, G., Young, D. C., Suarez, J. R., Colevas, A. D., & Grever, M. R. (2006). Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood, 109(2), 399–404. https://doi.org/10.1182/blood-2006-05-020735.
Byrd, J. C., Peterson, B. L., Gabrilove, J., Odenike, O. M., Grever, M. R., Rai, K., Larson, R. A., & Cancer and Leukemia Group B. (2005). Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from cancer and leukemia group B study 19805. Clinical Cancer Research, 11(11), 4176–4181. https://doi.org/10.1158/1078-0432.Ccr-04-2276.
Kouroukis, C. T., Belch, A., Crump, M., Eisenhauer, E., Gascoyne, R. D., Meyer, R., Lohmann, R., Lopez, P., Powers, J., Turner, R., Connors, J. M., & National Cancer Institute of Canada Clinical Trials Group. (2003). Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology, 21(9), 1740–1745. https://doi.org/10.1200/jco.2003.09.057.
Meijer, L., Borgne, A., Mulner, O., Chong, J. P. J., Blow, J. J., Inagaki, N., Inagaki, M., Delcros, J. G., & Moulinoux, J. P. (1997). Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. European Journal of Biochemistry, 243(1–2), 527–536. https://doi.org/10.1111/j.1432-1033.1997.t01-2-00527.x.
Whittaker, S. R., Walton, M. I., Garrett, M. D., & Workman, P. (2004). The cyclin-dependent kinase inhibitor CYC202 (R-Roscovitine) inhibits retinoblastoma protein phosphorylation, causes loss of Cyclin D1, and activates the mitogen-activated protein kinase pathway. Cancer Research, 64(1), 262–272. https://doi.org/10.1158/0008-5472.Can-03-0110.
McClue, S. J., Blake, D., Clarke, R., Cowan, A., Cummings, L., Fischer, P. M., MacKenzie, M., Melville, J., Stewart, K., Wang, S., Zhelev, N., Zheleva, D., & Lane, D. P. (2002). In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine). International Journal of Cancer, 102(5), 463–468. https://doi.org/10.1002/ijc.10738.
Benson, C., White, J., Bono, J. D., O'Donnell, A., Raynaud, F., Cruickshank, C., McGrath, H., Walton, M., Workman, P., Kaye, S., Cassidy, J., Gianella-Borradori, A., Judson, I., & Twelves, C. (2007). A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. British Journal of Cancer, 96(1), 29–37. https://doi.org/10.1038/sj.bjc.6603509.
Misra, R. N., **ao, H.-y., Kim, K. S., Lu, S., Han, W.-C., Barbosa, S. A., et al. (2004). N-(Cycloalkylamino)acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2. N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4- piperidinecarboxamide (BMS-387032), a highly efficacious and selective antitumor agent. Journal of Medicinal Chemistry, 47(7), 1719–1728. https://doi.org/10.1021/jm0305568.
Nuwayhid, S., Stockett, D., Hyde, J., Aleshin, A., Walker, D. H., & Arkin, M. R. SNS-032 is a potent and selective inhibitor of Cdk2, 7 and 9 and induces cell death by inhibiting cell cycle progression and the expression of antiapoptotic proteins. In Proc Am Assoc Cancer Res, 2006 (Vol. 47, pp. 491).
Tong, W.-G., Chen, R., Plunkett, W., Siegel, D., Sinha, R., Harvey, R. D., Badros, A. Z., Popplewell, L., Coutre, S., Fox, J. A., Mahadocon, K., Chen, T., Kegley, P., Hoch, U., & Wierda, W. G. (2010). Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. Journal of Clinical Oncology, 28(18), 3015–3022. https://doi.org/10.1200/jco.2009.26.1347.
Heath, E. I., Bible, K., Martell, R. E., Adelman, D. C., & LoRusso, P. M. (2008). A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Investigational New Drugs, 26(1), 59–65. https://doi.org/10.1007/s10637-007-9090-3.
Aklilu, M., Kindler, H. L., Donehower, R. C., Mani, S., & Vokes, E. E. (2003). Phase II study of flavopiridol in patients with advanced colorectal cancer. Annals of Oncology, 14(8), 1270–1273. https://doi.org/10.1093/annonc/mdg343.
Burdette-Radoux, S., Tozer, R. G., Lohmann, R. C., Quirt, I., Ernst, D. S., Walsh, W., Wainman, N., Colevas, A. D., & Eisenhauer, E. A. (2004). Phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Investigational New Drugs, 22(3), 315–322. https://doi.org/10.1023/B:DRUG.0000026258.02846.1c.
Le Tourneau, C., Faivre, S., Laurence, V., Delbaldo, C., Vera, K., Girre, V., et al. (2010). Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. European Journal of Cancer, 46(18), 3243–3250. https://doi.org/10.1016/j.ejca.2010.08.001.
Marra, A., & Curigliano, G. (2019). Are all cyclin-dependent kinases 4/6 inhibitors created equal? npj Breast Cancer, 5(1), 27. https://doi.org/10.1038/s41523-019-0121-y.
Hazel, P., Kroll, S. H. B., Bondke, A., Barbazanges, M., Patel, H., Fuchter, M. J., Coombes, R. C., Ali, S., Barrett, A. G. M., & Freemont, P. S. (2017). Inhibitor selectivity for cyclin-dependent kinase 7: a structural, thermodynamic, and Modelling study. ChemMedChem, 12(5), 372–380. https://doi.org/10.1002/cmdc.201600535.
Kelso, T. W., Baumgart, K., Eickhoff, J., Albert, T., Antrecht, C., Lemcke, S., et al. (2014). Cyclin-dependent kinase 7 controls mRNA synthesis by affecting stability of preinitiation complexes, leading to altered gene expression, cell cycle progression, and survival of tumor cells. Molecular and Cellular Biology, 34(19), 3675–3688. https://doi.org/10.1128/mcb.00595-14.
Choi, Y. J., Kim, D. H., Yoon, D. H., Suh, C., Choi, C.-M., Lee, J. C., Hong, J. Y., & Rho, J. K. (2019). Efficacy of the novel CDK7 inhibitor QS1189 in mantle cell lymphoma. Scientific Reports, 9(1), 7193. https://doi.org/10.1038/s41598-019-43760-z.
Wang, B. Y., Liu, Q. Y., Cao, J., Chen, J. W., & Liu, Z. S. (2016). Selective CDK7 inhibition with BS-181 suppresses cell proliferation and induces cell cycle arrest and apoptosis in gastric cancer. Drug Design, Development and Therapy, 10, 1181–1189. https://doi.org/10.2147/dddt.S86317.
Gong, Y., Yang, J., Liu, F., Li, Z., Gong, R., & Wei, T. (2018). Cyclin-dependent kinase 7 is a potential therapeutic target in papillary thyroid carcinoma. Journal of Biological Regulators and Homeostatic Agents, 32(6), 1361–1368.
Clark, K., Ainscow, E., Peall, A., Thomson, S., Leishman, A., Elaine, S., et al. (2017). CT7001, a novel orally bio-available CDK7 inhibitor, is highly active in in-vitro and in-vivo models of AML. Blood, 130(Suppl 1), 2645–2645.
Hutterer, C., Eickhoff, J., Milbradt, J., Korn, K., Zeittrager, I., Bahsi, H., et al. (2015). A novel CDK7 inhibitor of the pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations. Antimicrobial Agents and Chemotherapy, 59(4), 2062–2071. https://doi.org/10.1128/aac.04534-14.
Christensen, C. L., Kwiatkowski, N., Abraham, B. J., Carretero, J., Al-Shahrour, F., Zhang, T., et al. (2014). Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell, 26(6), 909–922. https://doi.org/10.1016/j.ccell.2014.10.019.
Cayrol, F., Praditsuktavorn, P., Fernando, T. M., Kwiatkowski, N., Marullo, R., Calvo-Vidal, M. N., Phillip, J., Pera, B., Yang, S. N., Takpradit, K., Roman, L., Gaudiano, M., Crescenzo, R., Ruan, J., Inghirami, G., Zhang, T., Cremaschi, G., Gray, N. S., & Cerchietti, L. (2017). THZ1 targeting CDK7 suppresses STAT transcriptional activity and sensitizes T-cell lymphomas to BCL2 inhibitors. Nature Communications, 8(1), 14290. https://doi.org/10.1038/ncomms14290.
Nagaraja, S., Vitanza, N. A., Woo, P. J., Taylor, K. R., Liu, F., Zhang, L., et al. (2017). Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell, 31(5), 635–652.e636. https://doi.org/10.1016/j.ccell.2017.03.011.
Greenall, S. A., Lim, Y. C., Mitchell, C. B., Ensbey, K. S., Stringer, B. W., Wilding, A. L., O'Neill, G. M., McDonald, K. L., Gough, D. J., Day, B. W., & Johns, T. G. (2017). Cyclin-dependent kinase 7 is a therapeutic target in high-grade glioma. Oncogenesis, 6(5), e336. https://doi.org/10.1038/oncsis.2017.33.
Eliades, P., Abraham, B. J., Ji, Z., Miller, D. M., Christensen, C. L., Kwiatkowski, N., Kumar, R., Njauw, C. N., Taylor, M., Miao, B., Zhang, T., Wong, K. K., Gray, N. S., Young, R. A., & Tsao, H. (2018). High MITF expression is associated with super-enhancers and suppressed by CDK7 inhibition in melanoma. Journal of Investigative Dermatology, 138(7), 1582–1590. https://doi.org/10.1016/j.jid.2017.09.056.
Zhong, L., Yang, S., Jia, Y., & Lei, K. (2018). Inhibition of cyclin-dependent kinase 7 suppresses human hepatocellular carcinoma by inducing apoptosis. Journal of Cellular Biochemistry, 119(12), 9742–9751. https://doi.org/10.1002/jcb.27292.
Cao, X., Dang, L., Zheng, X., Lu, Y., Lu, Y., Ji, R., Zhang, T., Ruan, X., Zhi, J., Hou, X., Yi, X., Li, M. J., Gu, T., Gao, M., Zhang, L., & Chen, Y. (2019). Targeting super-enhancer-driven oncogenic transcription by CDK7 inhibition in anaplastic thyroid carcinoma. Thyroid, 29(6), 809–823. https://doi.org/10.1089/thy.2018.0550.
Lu, P., Geng, J., Zhang, L., Wang, Y., Niu, N., Fang, Y., Liu, F., Shi, J., Zhang, Z. G., Sun, Y. W., Wang, L. W., Tang, Y., & Xue, J. (2019). THZ1 reveals CDK7-dependent transcriptional addictions in pancreatic cancer. Oncogene, 38(20), 3932–3945. https://doi.org/10.1038/s41388-019-0701-1.
Zhong, S., Zhang, Y., Yin, X., & Di, W. (2019). CDK7 inhibitor suppresses tumor progression through blocking the cell cycle at the G2/M phase and inhibiting transcriptional activity in cervical cancer. OncoTargets and Theraphy, 12, 2137–2147. https://doi.org/10.2147/ott.S195655.
Wang, Y., Zhang, T., Kwiatkowski, N., Abraham, B. J., Lee, T. I., **e, S., Yuzugullu, H., von, T., Li, H., Lin, Z., Stover, D. G., Lim, E., Wang, Z. C., Iglehart, J. D., Young, R. A., Gray, N. S., & Zhao, J. J. (2015). CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell, 163(1), 174–186. https://doi.org/10.1016/j.cell.2015.08.063.
Zhang, Y., Zhou, L., Bandyopadhyay, D., Sharma, K., Allen, A. J., Kmieciak, M., & Grant, S. (2019). The covalent CDK7 inhibitor THZ1 potently induces apoptosis in multiple myeloma cells in vitro and in vivo. Clinical Cancer Research, 25, 6195–6205. https://doi.org/10.1158/1078-0432.Ccr-18-3788.
Harrod, A., Fulton, J., Nguyen, V. T. M., Periyasamy, M., Ramos-Garcia, L., Lai, C. F., et al. (2016). Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene, 36, 2286, https://doi.org/10.1038/onc.2016.382https://www.nature.com/articles/onc2016382#supplementary-information.
Durbin, A. D., Zimmerman, M. W., Dharia, N. V., Abraham, B. J., Iniguez, A. B., Weichert-Leahey, N., He, S., Krill-Burger, J. M., Root, D. E., Vazquez, F., Tsherniak, A., Hahn, W. C., Golub, T. R., Young, R. A., Look, A. T., & Stegmaier, K. (2018). Selective gene dependencies in MYCN-amplified neuroblastoma include the core transcriptional regulatory circuitry. Nature Genetics, 50(9), 1240–1246. https://doi.org/10.1038/s41588-018-0191-z.
Huang, T., Ding, X., Xu, G., Chen, G., Cao, Y., Peng, C., Shen, S., Lv, Y., Wang, L., & Zou, X. (2019). CDK7 inhibitor THZ1 inhibits MCL1 synthesis and drives cholangiocarcinoma apoptosis in combination with BCL2/BCL-XL inhibitor ABT-263. Cell Death & Disease, 10(8), 602. https://doi.org/10.1038/s41419-019-1831-7.
Kalan, S., Amat, R., Schachter, M. M., Kwiatkowski, N., Abraham, B. J., Liang, Y., Zhang, T., Olson, C. M., Larochelle, S., Young, R. A., Gray, N. S., & Fisher, R. P. (2017). Activation of the p53 transcriptional program sensitizes cancer cells to Cdk7 inhibitors. Cell Reports, 21(2), 467–481. https://doi.org/10.1016/j.celrep.2017.09.056.
Huang, J.-R., Qin, W.-M., Wang, K., Fu, D.-R., Zhang, W.-J., Jiang, Q.-W., Yang, Y., Yuan, M. L., **ng, Z. H., Wei, M. N., Li, Y., & Shi, Z. (2018). Cyclin-dependent kinase 7 inhibitor THZ2 inhibits the growth of human gastric cancer in vitro and in vivo. American Journal of Translational Research, 10(11), 3664–3676.
Olson, C. M., Liang, Y., Leggett, A., Park, W. D., Li, L., Mills, C. E., Elsarrag, S. Z., Ficarro, S. B., Zhang, T., Düster, R., Geyer, M., Sim, T., Marto, J. A., Sorger, P. K., Westover, K. D., Lin, C. Y., Kwiatkowski, N., & Gray, N. S. (2019). Development of a selective CDK7 covalent inhibitor reveals predominant cell-cycle phenotype. Cell Chemical Biology, 26, 792–803.e10. https://doi.org/10.1016/j.chembiol.2019.02.012.
Zhang, H., Christensen, C. L., Dries, R., Oser, M. G., Deng, J., Diskin, B., et al. (2020). CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell, 37(1), 37–54.e39. https://doi.org/10.1016/j.ccell.2019.11.003.
Zhang, T., Kwiatkowski, N., Olson, C. M., Dixon-Clarke, S. E., Abraham, B. J., Greifenberg, A. K., et al. (2016). Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nature Chemical Biology, 12, 876. https://doi.org/10.1038/nchembio.2166https://www.nature.com/articles/nchembio.2166#supplementary-information.
MacCallum, D. E., Melville, J., Frame, S., Watt, K., Anderson, S., Gianella-Borradori, A., Lane, D. P., & Green, S. R. (2005). Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II–dependent transcription and down-regulation of Mcl-1. Cancer Research, 65(12), 5399–5407. https://doi.org/10.1158/0008-5472.Can-05-0233.
Shandilya, J., Wang, Y., & Roberts, S. G. E. (2012). TFIIB dephosphorylation links transcription inhibition with the p53-dependent DNA damage response. Proceedings of the National Academy of Sciences, 109(46), 18797–18802. https://doi.org/10.1073/pnas.1207483109.
Wong, R. W. J., Ngoc, P. C. T., Leong, W. Z., Yam, A. W. Y., Zhang, T., Asamitsu, K., Iida, S., Okamoto, T., Ueda, R., Gray, N. S., Ishida, T., & Sanda, T. (2017). Enhancer profiling identifies critical cancer genes and characterizes cell identity in adult T-cell leukemia. Blood, 130(21), 2326–2338. https://doi.org/10.1182/blood-2017-06-792184.
Sharifnia, T., Wawer, M. J., Chen, T., Huang, Q.-Y., Weir, B. A., Sizemore, A., Lawlor, M. A., Goodale, A., Cowley, G. S., Vazquez, F., Ott, C. J., Francis, J. M., Sassi, S., Cogswell, P., Sheppard, H. E., Zhang, T., Gray, N. S., Clarke, P. A., Blagg, J., Workman, P., Sommer, J., Hornicek, F., Root, D. E., Hahn, W. C., Bradner, J. E., Wong, K. K., Clemons, P. A., Lin, C. Y., Kotz, J. D., & Schreiber, S. L. (2019). Small-molecule targeting of brachyury transcription factor addiction in chordoma. Nature Medicine, 25(2), 292–300. https://doi.org/10.1038/s41591-018-0312-3.
Rasool, R. U., Natesan, R., Deng, Q., Aras, S., Lal, P., Sander Effron, S., et al. (2019). CDK7 inhibition suppresses castration-resistant prostate cancer through MED1 inactivation. Cancer Discovery, CD-19-0189. https://doi.org/10.1158/2159-8290.Cd-19-0189.
Jeselsohn, R., Bergholz, J. S., Pun, M., Cornwell, M., Liu, W., Nardone, A., et al. (2018). Allele-specific chromatin recruitment and therapeutic vulnerabilities of ESR1 activating mutations. Cancer Cell, 33(2), 173–186.e175. https://doi.org/10.1016/j.ccell.2018.01.004.
Sun, B., Mason, S., Wilson, R. C., Hazard, S. E., Wang, Y., Fang, R., Wang, Q., Yeh, E. S., Yang, M., Roberts, T. M., Zhao, J. J., & Wang, Q. (2019). Inhibition of the transcriptional kinase CDK7 overcomes therapeutic resistance in HER2-positive breast cancers. Oncogene, 39, 50–63. https://doi.org/10.1038/s41388-019-0953-9.
Martin, L.-A., Pancholi, S., Ribas, R., Gao, Q., Simigdala, N., Nikitorowicz-Buniak, J., et al. (2017). Abstract P3-03-09: resistance to palbociclib depends on multiple targetable mechanisms highlighting the potential of drug holidays and drug switching to improve therapeutic outcome. Cancer Research, 77(4 Supplement), P3-03-09-P03-03-09. https://doi.org/10.1158/1538-7445.Sabcs16-p3-03-09.
Alqahtani, A., Choucair, K., Ashraf, M., Hammouda, D. M., Alloghbi, A., Khan, T., Senzer, N., & Nemunaitis, J. (2019). Bromodomain and extra-terminal motif inhibitors: a review of preclinical and clinical advances in cancer therapy. Future Science OA, 5(3), FSO372. https://doi.org/10.4155/fsoa-2018-0115.
Gao, Y., Zhang, T., Terai, H., Ficarro, S. B., Kwiatkowski, N., Hao, M.-F., et al. (2018). Overcoming resistance to the THZ series of covalent transcriptional CDK inhibitors. Cell Chemical Biology, 25(2), 135–142.e135. https://doi.org/10.1016/j.chembiol.2017.11.007.
Sava, G. P., Fan, H., Fisher, R. A., Lusvarghi, S., Pancholi, S., Ambudkar, S. V., Martin, L. A., Charles Coombes, R., Buluwela, L., & Ali, S. (2019). ABC-transporter upregulation mediates resistance to the CDK7 inhibitors THZ1 and ICEC0942. Oncogene, 39, 651–663. https://doi.org/10.1038/s41388-019-1008-y.
Robey, R. W., Pluchino, K. M., Hall, M. D., Fojo, A. T., Bates, S. E., & Gottesman, M. M. (2018). Revisiting the role of ABC transporters in multidrug-resistant cancer. Nature Reviews Cancer, 18(7), 452–464. https://doi.org/10.1038/s41568-018-0005-8.
Kaliszczak, M., Patel, H., Kroll, S. H. B., Carroll, L., Smith, G., Delaney, S., Heathcote, D. A., Bondke, A., Fuchter, M. J., Coombes, R. C., Barrett, A. G. M., Ali, S., & Aboagye, E. O. (2013). Development of a cyclin-dependent kinase inhibitor devoid of ABC transporter-dependent drug resistance. British Journal of Cancer, 109(9), 2356–2367. https://doi.org/10.1038/bjc.2013.584.
Funding
The authors’ work is generously funded by Cancer Research UK (grant C37/A18784). Additional support was provided by the Imperial Experimental Cancer Medicine Centre, the Imperial NIHR Biomedical Research Centre and the Cancer Research UK Imperial Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RCC and SA are named as inventors on CDK7 inhibitor patents and own shares in Carrick Therapeutics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sava, G.P., Fan, H., Coombes, R.C. et al. CDK7 inhibitors as anticancer drugs. Cancer Metastasis Rev 39, 805–823 (2020). https://doi.org/10.1007/s10555-020-09885-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09885-8