Abstract
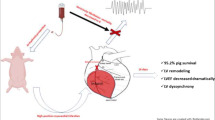
Most animal models use surgical thoracotomy with ligation of a coronary artery to induce myocardial infarction. Incision of the chest wall and myocardium affect remodeling after myocardial infarction. The aim of our study was to evaluate a new minimally invasive technique for inducing acute myocardial infarction in pigs. To this end, coronary angiography using a 6-F cardiac catheter was performed in 20 pigs. The cardiac catheter was advanced into the left circumflex artery (LCX) under electrocardiographic monitoring and small tungsten spirals were deployed in the vessel. LCX occlusion was verified by coronary angiography. Two days later, magnetic resonance imaging (MRI) was performed to estimate the extent of infarction. Thereafter, all animals were euthanized and the hearts stained with 2,3,5-triphenyltetrazolium chloride for histologic measurement of infarct size. Tungsten spirals were successfully placed in the LCX in all 20 pigs. About 13 of the 20 animals survived until the end of the experiment. The mean infarct size in the area supplied by the LCX was 4.4 ± 2.3 cm3 at MRI and 4.3 ± 2.2 cm3 at histology (r = 0.99, P < 0.001). No other myocardial regions showed infarction in any of the 13 pigs. Five of nine pigs requiring defibrillation due to ventricular fibrillation died because defibrillation was unsuccessful. One animal each died from pericarditis and pneumonia. Our results show that the minimally invasive method presented here enables reliable induction of myocardial infarction in a fairly straightforward manner. The 25% mortality rate associated with induction of myocardial infarction in our study is comparable to that reported by other investigators.


Similar content being viewed by others
Abbreviations
- LCX:
-
Left circumflex artery
- MRI:
-
Magnetic resonance imaging
- TTC-2,3,5:
-
Triphenyltetrazolium chloride
- CHD:
-
Coronary heart disease
- CT:
-
Computed tomography
- ECG:
-
Electrocardiography
- IR-FLASH:
-
Inversion recovery fast low angle shot
References
Ho KK, Pinsky JL, Kannel WB, Levy D (1993) The epidemiology of heart failure: the framingham study. J Am Coll Cardiol 22:6A–13A
Dewey M, Hamm B (2003) Cost-effectiveness in diagnosis of coronary artery disease. Rofo 175:749–751
Flohr TG, Ohnesorge BM (2008) Imaging of the heart with computed tomography. Basic Res Cardiol 103:161–173. doi:10.1007/s00395-008-0699-y
Bordeleau E, Lamonde A, Prenovault J et al (2007) Accuracy and rate of coronary artery segment visualization with CT angiography for the non-invasive detection of coronary artery stenoses. Int J Cardiovasc Imaging 23:771–780. doi:10.1007/s10554-006-9198-0
Hoe JW, Toh KH (2007) A practical guide to reading CT coronary angiograms—how to avoid mistakes when assessing for coronary stenoses. Int J Cardiovasc Imaging 23:617–633. doi:10.1007/s10554-006-9173-9
Fuster V, Kim RJ (2005) Frontiers in cardiovascular magnetic resonance. Circulation 112:135–144. doi:10.1161/01.CIR.0000155618.37779.A0
Futamatsu H, Klassen C, Pilla M et al (2008) Diagnostic accuracy of quantitative cardiac MRI evaluation compared to stress single-photon-emission computed tomography. Int J Cardiovasc Imaging 24:293–299. doi:10.1007/s10554-007-9263-3
Nagel E, Klein C, Paetsch I et al (2003) Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation 108:432–437. doi:10.1161/01.CIR.0000080915.35024.A9
Baur LH (2008) Cardiac imaging at the emergency department is a must! The role of cardiac computed tomography and magnetic resonance imaging in the evaluation of acute chest pain in the emergency department. Int J Cardiovasc Imaging 24:343–344. doi:10.1007/s10554-007-9280-2
Power JM, Tonkin AM (1999) Large animal models of heart failure. Aust N Z J Med 29:395–402
Christian TF, Peters K, Keck B, Allen J, Owens T, Borah B (2008) Gated SPECT imaging to detect changes in myocardial blood flow during progressive coronary occlusion. Int J Cardiovasc Imaging 24:269–276. doi:10.1007/s10554-007-9255-3
Fozzard HA (1975) Validity of myocardial infarction models. Circulation 52:III131–III146
Bjerner T, Johansson L, Wikstrom G et al (2004) In and ex vivo MR evaluation of acute myocardial ischemia in pigs by determining R1 in steady state after the administration of the intravascular contrast agent NC100150 injection. Invest Radiol 39:479–486. doi:10.1097/01.rli.0000128658.63611.b3
Krombach GA, Kinzel S, Mahnken AH, Gunther RW, Buecker A (2005) Minimally invasive close-chest method for creating reperfused or occlusive myocardial infarction in swine. Invest Radiol 40:14–18
Ruzsics B, Suranyi P, Kiss P et al (2009) Myocardial strain in sub-acute peri-infarct myocardium. Int J Cardiovasc Imaging 25:151–159. doi:10.1007/s10554-008-9364-7
Weismuller P, Mayer U, Richter P, Heieck F, Kochs M, Hombach V (1991) Chemical ablation by subendocardial injection of ethanol via catheter—preliminary results in the pig heart. Eur Heart J 12:1234–1239
Haines DE, Whayne JG, DiMarco JP (1994) Intracoronary ethanol ablation in swine: effects of ethanol concentration on lesion formation and response to programmed ventricular stimulation. J Cardiovasc Electrophysiol 5:422–431. doi:10.1111/j.1540-8167.1994.tb01181.x
White FC, Roth DM, Bloor CM (1989) Coronary collateral reserve during exercise induced ischemia in swine. Basic Res Cardiol 84:42–54. doi:10.1007/BF01907002
Weaver ME, Pantely GA, Bristow JD, Ladley HD (1986) A quantitative study of the anatomy and distribution of coronary arteries in swine in comparison with other animals and man. Cardiovasc Res 20:907–917. doi:10.1093/cvr/20.12.907
Fishbein MC, Meerbaum S, Rit J et al (1981) Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J 101:593–600. doi:10.1016/0002-8703(81)90226-X
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Jugdutt BI, Tang SB, Khan MI, Basualdo CA (1992) Functional impact of remodeling during healing after non-Q wave versus Q wave anterior myocardial infarction in the dog. J Am Coll Cardiol 20:722–731
Schwarz ER, Fleischhauer J, Montino H et al (1998) Infarct size reduction by ischemic preconditioning is a monophasic, short-lived phenomenon in anesthetized pigs. J Cardiovasc Pharmacol Ther 3:63–70. doi:10.1177/107424849800300108
Schwarz ER, Reffelmann T, Schoendube F et al (1999) Hypoxic hypoperfusion fails to induce myocardial hibernation in anesthetized swine. J Cardiovasc Pharmacol Ther 4:235–247. doi:10.1177/107424849900400405
Schulz R, Post H, Vahlhaus C, Heusch G (1998) Ischemic preconditioning in pigs: a graded phenomenon: its relation to adenosine and bradykinin. Circulation 98:1022–1029
Trolese-Mongheal Y, Duchene-Marullaz P, Trolese JF, Leinot M, Lamar JC, Lacroix P (1985) Sudden death and experimental acute myocardial infarction. Am J Cardiol 56:677–681. doi:10.1016/0002-9149(85)91034-3
White FC, Roth DM, Bloor CM (1986) The pig as a model for myocardial ischemia and exercise. Lab Anim Sci 36:351–356
Kraitchman DL, Bluemke DA, Chin BB, Heldman AW (2000) A minimally invasive method for creating coronary stenosis in a swine model for MRI and SPECT imaging. Invest Radiol 35:445–451. doi:10.1097/00004424-200007000-00008
Hosenpud JD, Yung NN, Morton MJ (1983) Left ventricular pressure-volume relations shift to the left after long-term loss of pericardial restraint. Circulation 68:155–163
Wang SY, Sheldon RS, Bergman DW, Tyberg JV (1995) Effects of pericardial constraint on left ventricular mechanoreceptor activity in cats. Circulation 92:3331–3336
Robotham JL, Stuart RS, Borkon AM, Doherty K, Baumgartner W (1988) Effects of changes in left ventricular loading and pleural pressure on mitral flow. J Appl Physiol 65:1662–1675
Grund F, Sommerschild HT, Kirkeboen KA, Ilebekk A (1998) A new approach to normalize myocardial temperature in the open-chest pig model. J Appl Physiol 84:2190–2197
Hale SL, Dave RH, Kloner RA (1997) Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol 92:351–357
Bitkover CY, Hansson LO, Valen G, Vaage J (2000) Effects of cardiac surgery on some clinically used inflammation markers and procalcitonin. Scand Cardiovasc J 34:307–314. doi:10.1080/713783128
Eldar M, Ohad D, Bor A, Varda-Bloom N, Swanson DK, Battler A (1994) A closed-chest pig model of sustained ventricular tachycardia. Pacing Clin Electrophysiol 17:1603–1609. doi:10.1111/j.1540-8159.1994.tb02353.x
Skyschally A, Leineweber K, Gres P, Haude M, Erbel R, Heusch G (2006) Coronary microembolization. Basic Res Cardiol 101:373–382. doi:10.1007/s00395-006-0616-1
Suzuki M, Asano H, Tanaka H, Usuda S (1999) Development and evaluation of a new canine myocardial infarction model using a closed-chest injection of thrombogenic material. Jpn Circ J 63:900–905. doi:10.1253/jcj.63.900
Reffelmann T, Sensebat O, Birnbaum Y et al (2004) A novel minimal-invasive model of chronic myocardial infarction in swine. Coron Artery Dis 15:7–12. doi:10.1097/00019501-200402000-00002
Naslund U, Haggmark S, Johansson G, Marklund SL, Reiz S (1992) A closed-chest myocardial occlusion-reperfusion model in the pig: techniques, morbidity and mortality. Eur Heart J 13:1282–1289
Lumb G, Hardy LB (1963) Collateral Circulation in the Heart. N C Med J 24:456–460
Klein HH, Schubothe M, Nebendahl K, Kreuzer H (1984) Temporal and spatial development of infarcts in porcine hearts. Basic Res Cardiol 79:440–447. doi:10.1007/BF01908144
Schaper W, Gorge G, Winkler B, Schaper J (1988) The collateral circulation of the heart. Prog Cardiovasc Dis 31:57–77. doi:10.1016/0033-0620(88)90011-4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peukert, D., Laule, M., Kaufels, N. et al. A minimally invasive method for induction of myocardial infarction in an animal model using tungsten spirals. Int J Cardiovasc Imaging 25, 529–535 (2009). https://doi.org/10.1007/s10554-009-9442-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-009-9442-5