Abstract
Purpose
Breast cancers have a poorer prognosis if estrogen receptor expression was lost during recurrence. It is unclear whether this conversion is cell autonomous or whether it can be promoted by the microenvironment during cancer dormancy. We explored the ability of marrow-derived stromal cell lines to arrest co-cultured breast cancer cells and suppress estrogen receptor alpha (ER) expression during arrest, facilitating the emergence of estrogen-independent breast cancer clones.
Methods
Cancer cell growth, ER protein, microRNA, and mRNA levels were measured in breast cancer cell lines exposed to conditioned medium from marrow stromal lines in the presence and absence of estrogen and of signaling pathway modulators.
Results
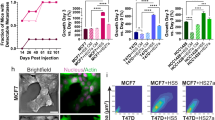
We demonstrate that paracrine signaling from the stromal cell line HS5 downregulated ER in T47D and MCF7 breast cancer cells. This occurred at the mRNA level and also through decreased ER protein stability. Additionally, conditioned medium (CM) from HS5 arrested the breast cancer cells in G0/G1 in part through interleukin-1 (IL1) and inhibited cancer cell growth despite the activation of proliferative pathways (Erk and AKT) by the CM. Similar findings were observed for CM from the hFOB 1.19 osteoblastic cell line but not from two other fibroblastic marrow lines, HS27A and KM101. HS5-CM inhibition of MCF7 proliferation could not be restored by exogenous ER, but was restored by the IL1-antagonist IL1RA. In the presence of IL1RA, HS5-CM activation of AKT and Erk enabled the outgrowth of breast cancer cells with suppressed ER that were fulvestrant-resistant and estrogen-independent.
Conclusions
We conclude that marrow-derived stromal cells can destabilize estrogen receptor protein to convert the ER status of growth-arrested ER+ breast cancer cell lines. The balance between stromal pro- and anti-proliferative signals controlled the switch from a dormant phenotype to estrogen-independent cancer cell growth.







Similar content being viewed by others
References
Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J et al (2010) Receptor conversion in distant breast cancer metastases. Breast Cancer Res 12(5):R75
Hoefnagel LD, Moelans CB, Meijer SL, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID et al (2012) Prognostic value of estrogen receptor alpha and progesterone receptor conversion in distant breast cancer metastases. Cancer 118(20):4929–4935
Fehm T, Krawczyk N, Solomayer EF, Becker-Pergola G, Durr-Storzer S, Neubauer H, Seeger H, Staebler A, Wallwiener D, Becker S (2008) ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res 10(5):R76
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7(332):ra63
Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP, Jedlicka P, Joensuu K, Heikkila P, Horwitz KB (2014) Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res 16(6):489
Zhou W, Slingerland JM (2014) Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat Rev Cancer 14(1):26–38
Macaluso M, Montanari M, Noto PB, Gregorio V, Bronner C, Giordano A (2007) Epigenetic modulation of estrogen receptor-α by pRb family proteins: a novel mechanism in breast cancer. Cancer Res 67(16):7731–7737
Dhasarathy A, Kajita M, Wade PA (2007) The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-α. Mol Endocrinol 21(12):2907–2918
Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao Y, Chen Y, Zhang PJ, Yu M, Zhen C et al (2011) Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nat Med 17(6):708–714
Fan M, Bigsby RM, Nephew KP (2003) The NEDD8 pathway is required for proteasome-mediated degradation of human estrogen receptor (ER)-alpha and essential for the antiproliferative activity of ICI 182,780 in ERα-positive breast cancer cells. Mol Endocrinol 17(3):356–365
Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW (1999) Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96(5):1858–1862
Klinge CM (2012) miRNAs and estrogen action. Trends Endocrinol Metab 23(5):223–233
Shah SH, Miller P, Garcia-Contreras M, Ao Z, Machlin L, Issa E, El-Ashry D (2015) Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK-microRNAs to drive ER-negative breast cancer phenotype. Cancer Biol Ther 16(11):1671–1681
Pinzone JJ, Stevenson H, Strobl JS, Berg PE (2004) Molecular and cellular determinants of estrogen receptor α expression. Mol Cell Biol 24(11):4605–4612
Sflomos G, Dormoy V, Metsalu T, Jeitziner R, Battista L, Scabia V, Raffoul W, Delaloye JF, Treboux A, Fiche M et al (2016) A preclinical model for ERα-positive breast cancer points to the epithelial microenvironment as determinant of luminal phenotype and hormone response. Cancer Cell 29(3):407–422
Roecklein BA, Torok-Storb B (1995) Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood 85(4):997–1005
Iwata M, Sandstrom RS, Delrow JJ, Stamatoyannopoulos JA, Torok-Storb B (2014) Functionally and phenotypically distinct subpopulations of marrow stromal cells are fibroblast in origin and induce different fates in peripheral blood monocytes. Stem Cells Dev 23(7):729–740
Iwata M, Torok-Storb B, Wayner EA, Carter WG (2014) CDCP1 identifies a CD146 negative subset of marrow fibroblasts involved with cytokine production. PLoS ONE 9(10):e109304
Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, Mariotti V, Buchupalli B, Foster K, Bonnet D et al (2013) A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res 73(23):6886–6899
Cavnar SP, Rickelmann AD, Meguiar KF, **ao A, Dosch J, Leung BM, Cai Lesher-Perez S, Chitta S, Luker KE, Takayama S et al (2015) Modeling selective elimination of quiescent cancer cells from bone marrow. Neoplasia 17(8):625–633
FitzGerald TJ, Santucci MA, Harigaya K, Woda B, McKenna M, Sakakeeny MA, Pierce JH, Kase K, Holland CA, Greenberger JS (1988) Radiosensitivity of permanent human bone marrow stromal cell lines: effect of dose rate. Int J Radiat Oncol Biol Phys 15(5):1153–1159
Kruger S, Abd Elmageed ZY, Hawke DH, Worner PM, Jansen DA, Abdel-Mageed AB, Alt EU, Izadpanah R (2014) Molecular characterization of exosome-like vesicles from breast cancer cells. BMC Cancer 14:44
Shelke GV, Lasser C, Gho YS, Lotvall J (2014) Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles 3:24783
Kobayashi A, Okuda H, **ng F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C et al (2011) Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med 208(13):2641–2655
Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L (2003) ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res 63(7):1684–1695
Chery L, Lam HM, Coleman I, Lakely B, Coleman R, Larson S, Aguirre-Ghiso JA, **a J, Gulati R, Nelson PS et al (2014) Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5(20):9939–9951
Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA (2011) ERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res 17(18):5850–5857
Paciotti GF, Tamarkin L (1988) Interleukin-1 directly regulates hormone-dependent human breast cancer cell proliferation in vitro. Mol Endocrinol 2(5):459–464
Cowland JB, Muta T, Borregaard N (2006) IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol 176(9):5559–5566
Harris SA, Enger RJ, Riggs BL, Spelsberg TC (1995) Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res 10(2):178–186
Stoica A, Saceda M, Doraiswamy VL, Coleman C, Martin MB (2000) Regulation of estrogen receptor-α gene expression by epidermal growth factor. J Endocrinol 165(2):371–378
Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB (2000) Role of insulin-like growth factor-I in regulating estrogen receptor-α gene expression. J Cell Biochem 76(4):605–614
West NR, Murphy LC, Watson PH (2012) Oncostatin M suppresses oestrogen receptor-α expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer 19(2):181–195
Chang HL, Sugimoto Y, Liu S, Ye W, Wang LS, Huang YW, Lin YC (2006) Keratinocyte growth factor (KGF) induces tamoxifen (Tam) resistance in human breast cancer MCF-7 cells. Anticancer Res 26(3A):1773–1784
Petrel TA, Brueggemeier RW (2003) Increased proteasome-dependent degradation of estrogen receptor-α by TGF-β1 in breast cancer cell lines. J Cell Biochem 88(1):181–190
Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, Ogura T, Makino H, Doihara H (2008) Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol 199(3):445–455
Danforth DN Jr, Sgagias MK (1991) Interleukin 1α blocks estradiol-stimulated growth and down-regulates the estrogen receptor in MCF-7 breast cancer cells in vitro. Cancer Res 51(5):1488–1493
Danforth DN Jr, Sgagias MK (1993) Interleukin-1α and interleukin-6 act additively to inhibit growth of MCF-7 breast cancer cells in vitro. Cancer Res 53(7):1538–1545
Lang JD, Berry SM, Powers GL, Beebe DJ, Alarid ET (2013) Hormonally responsive breast cancer cells in a microfluidic co-culture model as a sensor of microenvironmental activity. Integr Biol 5(5):807–816
Keshamouni VG, Mattingly RR, Reddy KB (2002) Mechanism of 17-β-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 AND PKC-delta. J Biol Chem 277(25):22558–22565
Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC, Landberg G (2005) ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene 24(27):4370–4379
Clark AS, West K, Streicher S, Dennis PA (2002) Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther 1(9):707–717
Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J et al (2015) The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27(2):193–210
Pinto MP, Badtke MM, Dudevoir ML, Harrell JC, Jacobsen BM, Horwitz KB (2010) Vascular endothelial growth factor secreted by activated stroma enhances angiogenesis and hormone-independent growth of estrogen receptor-positive breast cancer. Cancer Res 70(7):2655–2664
Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, Lacey MR et al (2010) Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat 121(2):293–300
Fuchs-Young R, Shirley SH, Lambertz I, Colby JK, Tian J, Johnston D, Gimenez-Conti IB, Donehower LA, Conti CJ, Hursting SD (2011) P53 genotype as a determinant of ER expression and tamoxifen response in the MMTV-Wnt-1 model of mammary carcinogenesis. Breast Cancer Res Treat 130(2):399–408
Sgagias MK, Kasid A, Danforth DN Jr (1991) Interleukin-1α and tumor necrosis factor-α (TNFα) inhibit growth and induce TNF messenger RNA in MCF-7 human breast cancer cells. Mol Endocrinol 5(11):1740–1747
Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM (2015) Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis 32(4):335–344
Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig 121(4):1298–1312
Chen Z, Orlowski RZ, Wang M, Kwak L, McCarty N (2014) Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood 123(14):2204–2208
Lawson MA, McDonald MM, Kovacic N, Hua Khoo W, Terry RL, Down J, Kaplan W, Paton-Hough J, Fellows C, Pettitt JA et al (2015) Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun 6:8983
Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL (2003) The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol 23(2):269–284
Kumar S, Kishimoto H, Chua HL, Badve S, Miller KD, Bigsby RM, Nakshatri H (2003) Interleukin-1α promotes tumor growth and cachexia in MCF-7 xenograft model of breast cancer. Am J Pathol 163(6):2531–2541
Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN (2003) IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA 100(5):2645–2650
Saito M, Fan D, Lachman LB (1995) Antitumor effects of liposomal IL1α and TNFα against the pulmonary metastases of the B16F10 murine melanoma in syngeneic mice. Clin Exp Metastasis 13(4):249–259
Pezzella KM, Neville ME, Huang JJ (1990) In vivo inhibition of tumor growth of B16 melanoma by recombinant interleukin 1β. I. Tumor inhibition parallels lymphocyte-activating factor activity of interleukin 1β proteins. Cytokine 2(5):357–362
Nakamura S, Nakata K, Kashimoto S, Yoshida H, Yamada M (1986) Antitumor effect of recombinant human interleukin 1α against murine syngeneic tumors. Jpn J Cancer Res 77(8):767–773
Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG (2012) The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150(4):764–779
Martinez J, Zhang XH (2013) BMP/Coco antagonism as a deterministic factor of metastasis dormancy in lung. Breast Cancer Res 15(1):302
Bodo M, Baroni T, Tabilio A (2009) Haematopoietic and stromal stem cell regulation by extracellular matrix components and growth factors. J Stem Cells 4(1):57–69
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat 123(3):725–731
Fernandez-Garcia B, Eiro N, Miranda MA, Cid S, Gonzalez LO, Dominguez F, Vizoso FJ (2016) Prognostic significance of inflammatory factors expression by stroma from breast carcinomas. Carcinogenesis 37(8):768–776
Mittempergher L, Saghatchian M, Wolf DM, Michiels S, Canisius S, Dessen P, Delaloge S, Lazar V, Benz SC, Tursz T et al (2013) A gene signature for late distant metastasis in breast cancer identifies a potential mechanism of late recurrences. Mol Oncol 7(5):987–999
Zhang XH, ** X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massague J (2013) Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154(5):1060–1073
Lewis AM, Varghese S, Xu H, Alexander HR (2006) Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med 4:48
Dinarello CA (2010) Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev 29(2):317–329
Triozzi PL, Aldrich W (2010) Effects of interleukin-1 receptor antagonist and chemotherapy on host-tumor interactions in established melanoma. Anticancer Res 30(2):345–354
Acknowledgements
This project was supported by NIH Grant R01CA154728 (RAS). The project used the UPCI Biostatistics Facility and Cancer Proteomics Facility: Luminex Core that are supported in part by award P30CA047904. We thank Drs. Steffi Oesterreich and Tim Burns (University of Pittsburgh) for helpful comments; Dr. Vera Donnenberg for assistance with flow cytometry and for HFF1 foreskin fibroblasts; and Lucas Santana-Santos for his work on the microRNA analysis algorithm. In addition, we appreciate the generosity of Didier Picard (Universite de Geneve) for the gift of the ER-3′-UTR reporter plasmid; Dr. Sarat Chandarlapaty (Sloan Kettering Cancer Center) for MCF7 cells stably expressing inducible estrogen receptor or vector (mCherry) control; and University of Pittsburgh investigators Steffi Oesterreich for T47D cells, Michael Epperly for KM101 cells, and Adrian Lee for phospho-IGF1R antibody. The 3X ERE TATA luc was a gift from Donald McDonnell (Addgene plasmid # 11354), and pEGFP-C1-ER alpha was a gift from Michael Mancini (Addgene plasmid # 28230). We thank Yingjian Li (University of Pittsburgh) for assistance in exosome preparation. The use of the fluorescence plate reader in the laboratory of Dr. Ben van Houten (University of Pittsburgh) is also appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors have conflicts of interest with any material in this report.
Additional information
J. Huang and P. Woods contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, J., Woods, P., Normolle, D. et al. Downregulation of estrogen receptor and modulation of growth of breast cancer cell lines mediated by paracrine stromal cell signals. Breast Cancer Res Treat 161, 229–243 (2017). https://doi.org/10.1007/s10549-016-4052-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4052-0