Abstract
Purpose
We examined acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) events among 9679 women treated for breast cancer on four adjuvant Alliance for Clinical Trials in Oncology trials with >90 months of follow-up in order to better characterize the risk for AML/MDS in older patients receiving anthracyclines.
Methods
We used multivariable Cox regression to examine factors associated with AML/MDS, adjusting for age (≥65 vs. <65 years; separately for ≥70 vs. <70 years), race/ethnicity, insurance, performance status, and anthracycline receipt. We also examined the effect of cyclophosphamide, the interaction of anthracycline and age, and outcomes for those develo** AML/MDS.
Results
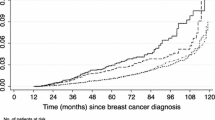
On Cancer and Leukemia Group B (CALGB) 40101, 49907, 9344, and 9741, 7290 received anthracyclines; 15% were in the age ≥65 and 7% were ≥70. Overall, 47 patients developed AML/MDS (30 AML [0.3%], 17 MDS [0.2%]); 83% of events occurred within 5 years of study registration. Among those age ≥65 and ≥70, 0.8 and 1.0% developed AML/MDS (vs. 0.4% for age <65), respectively. In adjusted analyses, older age and anthracycline receipt were significantly associated with AML/MDS (adjusted hazard ratio [HR] for age ≥65 [vs. <65] = 3.13, 95% confidence interval [CI] 1.18–8.33; HR for anthracycline receipt [vs. no anthracycline] = 5.16, 95% CI 1.47–18.19). There was no interaction between age and anthracycline use. Deaths occurred in 70% of those develo** AML/MDS.
Conclusions
We observed an increased risk for AML/MDS for older patients and those receiving anthracyclines, though these events were rare. Our results help inform discussions surrounding anticipated toxicities of adjuvant chemotherapy in older patients.
Similar content being viewed by others
References
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2015) SEER cancer statistics review, 1975–2012, National Cancer Institute. Bethesda. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site
Surveillance, Epidemiology, and End Results Cancer Statistics Review (1975–2013) Table 30.2: myelodysplastic syndromes, chronic myeloproliferative disorders, and chronic myelomonocytic leukemia, age-adjusted incidence rates for the 18 SEER geographic areas by age and race, 2009–2013. http://seer.cancer.gov/csr/1975_2013/browse_csr.php?sectionSEL=30&pageSEL=sect_30_table.02.html#table1. Accessed 28 July 2016
http://www.cancernetwork.com/cancer-management/mds. Accessed 7 Apr 2016
Ma X (2012) Epidemiology of myelodysplastic syndromes. Am J Med 125(7 Suppl):S2–5
Surveillance, Epidemiology, and End Results Program. Cancer Statistics Review. Table 13.13 myeloid leukmia. SEER incidene rates, age-adjusted and age-specific rates, by race and sex. http://seer.cancer.gov/csr/1975_2013/browse_csr.php?sectionSEL=13&pageSEL=sect_13_table.13.html#a. Accessed 28 July 2016
Wolff AC, Blackford AL, Visvanathan K, Rugo HS, Moy B, Goldstein LJ, Stockerl-Goldstein K, Neumayer L, Langbaum TS, Theriault RL et al (2015) Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol 33(4):340–348
Smith RE, Bryant J, DeCillis A, Anderson S, National Surgical Adjuvant B, Bowel Project E (2003) Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol 21(7):1195–1204
Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, Norton L, Winer EP, Hudis CA (2007) Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol 25(24):3699–3704
Le Deley MC, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, Linassier C, Clisant S, de Vathaire F, Fenaux P, Hill C (2007) Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol 25(3):292–300
Kaplan HG, Malmgren JA, Atwood MK (2011) Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC Cancer 11:260
Kaplan HG, Malmgren JA, Li CI, Calip GS (2013) Age related risk of myelodysplastic syndrome and acute myeloid leukemia among breast cancer survivors. Breast Cancer Res Treat 142(3):629–636
Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH (2007) Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol 25(25):3871–3876
Azim HA Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ (2011) Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol 22(9):1939–1947
Cox DR (1972) Regression models and life-tables. J R Stat Soc 34(2):187–220
Fine JP, Gray RJA (1999) Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444
Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS (2006) Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol 24(18):2750–2756
Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS (2006) Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: assessing outcome in a population-based, observational cohort. J Clin Oncol 24(18):2757–2764
Praga C, Bergh J, Bliss J, Bonneterre J, Cesana B, Coombes RC, Fargeot P, Folin A, Fumoleau P, Giuliani R et al (2005) Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 23(18):4179–4191
Tallman MS, Gray R, Bennett JM, Variakojis D, Robert N, Wood WC, Rowe JM, Wiernik PH (1995) Leukemogenic potential of adjuvant chemotherapy for early-stage breast cancer: the Eastern Cooperative Oncology Group experience. J Clin Oncol 13(7):1557–1563
Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, Wood WC, Henderson IC, Hudis C, Winer E et al (2005) Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA 293(9):1073–1081
Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V et al (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 29(25):3457–3465
Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ 3rd et al (2012) Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118(13):3377–3386
Acknowledgements
We thank all patients enrolled to Alliance trials over time as well as the study teams who previously collected and shared the data for analysis. We also thank Kaitlyn Bifolck for her administrative support with manuscript submission.
Funding
This study was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA032291, U10CA047559, U10CA047577, U10CA077597, U10CA077651, U10CA180790, U10CA180791, U10CA180838, U10CA180857, U10CA180867. RAF also receives funding Susan G. Komen (Grant No. CCR14298143) and American Cancer Society (Grant No. 125912-MRSG-14-240-01-CPPB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rachel Freeman has received institutional funding from Genentech, Puma, and Eisai. Drew Seisler reports renumeration from Mayo Clinic. Jared Foster declares that he has no conflict of interest. Jeff Sloan declares that he has no conflict of interest. Jacqueline Lafky declares that she has no conflict of interest. Gretchen Kimmick has served on speakers bureaus and has consultant/advisory roles for Pfizer, Astra Zeneca, Novartis, Genomic Health, and Genentech, and has received funding from Wyeth, Astra Zeneca, Glaxo Smith Kline, Novartis, PUMA, Bristol Meyers Squibb, and Bionovo. Arti Hurria has consultant/advisory roles for Boehringer Ingelheim Pharmaceuticals, Carevive, Sanofi, and GTx, Inc, and has received funding from Celegene, Novartis, and GSK. Harvey Cohen declares that he has no conflict of interest. Eric Winer declares that he has no conflict of interest. Clifford Hudis declares that he has no conflict of interest. Ann Partridge declares that she has no conflict of interest. Lisa Carey declares that she has no conflict of interest. Aminah Jatoi declares that he has no conflict of interest. Heidi Klepin declares that she has no conflict of interest. Marc Citron reports speakers bureau roles for Genentech and Pfizer, consultant/advisory roles for Genentech and Pfizer, and funding from Genentech, Pfizer, Celldex, Merck, Puma, and Macrogenics. Donald Berry reports renumeration from Berry Consultants, LLC, a consultant/advisory role for Berry Consultants, LLC, and stock ownership in Berry Consultants, LLC (co-owner). Lawrence Shulman declares that he has no conflict of interest. Aman Buzdar declares that he has no conflict of interest. Vera Suman declares that she has no conflict of interest. Hyman Muss declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The manuscript only includes secondary analysis of pre-existing data that were collected as part of the included clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Freedman, R.A., Seisler, D.K., Foster, J.C. et al. Risk of acute myeloid leukemia and myelodysplastic syndrome among older women receiving anthracycline-based adjuvant chemotherapy for breast cancer on Modern Cooperative Group Trials (Alliance A151511). Breast Cancer Res Treat 161, 363–373 (2017). https://doi.org/10.1007/s10549-016-4051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4051-1