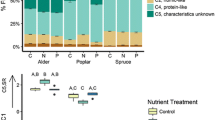
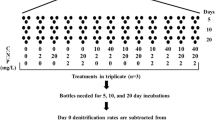
Abstract
Organic carbon supply is linked to nitrogen transformation in ecosystems. However, the role of organic carbon quality in nitrogen processing is not as well understood. We determined how the quality of particulate organic carbon (POC) influenced nitrogen transformation in stream sediments by burying identical quantities of varying quality POC (northern red oak (Quercus rubra) leaves, red maple (Acer rubrum) leaves, red maple wood) in stream mesocosms and measuring the effects on nitrogen retention and denitrification compared to a control of combusted sand. We also determined how POC quality affected the quantity and quality of dissolved organic carbon (DOC) and dissolved oxygen concentration in groundwater. Nitrate and total dissolved nitrogen (TDN) retention were assessed by comparing solute concentrations and fluxes along groundwater flow paths in the mesocosms. Denitrification was measured by in situ changes in N2 concentrations (using MIMS) and by acetylene block incubations. POC quality was measured by C:N and lignin:N ratios and DOC quality was assessed by fluorescence excitation emission matrix spectroscopy. POC quality had strong effects on nitrogen processing. Leaf treatments had much higher nitrate retention, TDN retention and denitrification rates than the wood and control treatments and red maple leaf burial resulted in higher nitrate and TDN retention rates than burial of red oak leaves. Leaf, but not wood, burial drove pore water to severe hypoxia and leaf treatments had higher DOC production and different DOC chemical composition than the wood and control treatments. We think that POC quality affected nitrogen processing in the sediments by influencing the quantity and quality of DOC and redox conditions. Our results suggest that the type of organic carbon inputs can affect the rates of nitrogen transformation in stream ecosystems.







Similar content being viewed by others
References
Alexander HD, Arthur MA (2010) Implications of a predicted shift from upland oaks to red maple on forest hydrology and nutrient availability. Can J For Res 40:716–726. doi:10.1139/X10-029
AOAC-973.18 (1990) Fiber (acid detergent) and lignin in animal feed. Official methods of analysis of the Association of Official Analytical Chemists, 15th edn. Arlington, Virginia
Arango CP, Tank JL, Schaller JM, Royer TV, Bernot MJ, David MB (2007) Benthic organic carbon influences denitrification in streams with high nitrate concentration. Freshw Biol 52:1210–1222. doi:10.1111/j.1365-2427.2007.01758.x
Arango CP, Tank JL, Johnson LT, Hamilton SK (2008) Assimilatory uptake rather than nitrification and denitrification determines nitrogen removal patterns in streams of varying land use. Limnol Oceanogr 53:2558–2572. doi:10.4319/lo.2008.53.6.2558
Attard E, Recous S, Chabbi A, De Berranger C, Guillaumaud N, Labreuche J, Philippot L, Schmid B, Le Roux X (2011) Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land uses. Glob Change Biol 17:1975–1989. doi:10.1111/j.1365-2486.2010.02340.x
Baker MA, Vervier P (2004) Hydrological variability, organic matters supply and denitrification in the Garonne River ecosystem. Freshw Biol 49:181–190
Barnes RT, Smith RL, Aiken GR (2012) Linkages between denitrification and dissolved organic matter quality, Boulder Creek Watershed, Colorado. J Geophys Res 117:G01014. doi:10.1029/2011JG001749
Bastviken SK, Eriksson PG, Premrov A, Tonderski K (2005) Potential denitrification in wetland sediments with different plant species detritus. Ecol Eng 25:183–190. doi:10.1016/j.ecoleng.2005.04.013
Battin TJ, Kaplan LA, Findlay S, Hopkinson CS, Marti E, Packman AI, Newbold JD, Sabater F (2009) Biophysical controls on organic carbon fluxes in fluvial networks. Nat Geosci 1:95–100. doi:10.1038/ngeo101
Beaulieu JJ et al (2011) Nitrous oxide emission from denitrification in stream and river networks. Proc Natl Acad Sci 108:214–219. doi:10.1073/pnas.1011464108
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13. doi:10.1111/j.1574-6941.2009.00654.x
Bro R, Kiers HAL (2003) A new efficient method for determining the number of components in PARAFAC models. J Chemom 17:274–286. doi:10.1002/cem.801
Cheever BM, Kratzer EB, Webster JR (2012) Immobilization and mineralization of N and P by heterotrophic microbes during leaf decomposition. Freshwater Sci 31:133–147. doi:10.1899/11-060.1
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar Chem 51:325–346. doi:10.1016/0304-4203(95)00062-3
Cornut J, Elger A, Lambrigot D, Marmonier P (2010) Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshw Biol 55:2541–2556. doi:10.1111/j.1365-2427.2010.02483.x
Cory RM, Kaplan LA (2012) Biological lability of streamwater fluorescent dissolved organic matter. Limnol Oceanogr 57:1347–1360. doi:10.4319/lo.2012.57.5.1347
Cory RM, McKnight DM (2005) Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ Sci Technol 39:8142–8149. doi:10.1021/es0506962
Dahm CN, Valett HM, Baxter CV, Woessner WW (2006) Hyporheic zones. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology. Elsevier, Boston, pp 119–142
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Dodla SK, Wang JJ, DeLaune RD, Cook RL (2008) Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci Total Environ 407:471–480. doi:10.1016/j.scitotenv.2008.08.022
Duff JH, Murphy F, Fuller CC, Triska FJ, Harvey JW, Jackman AR (1998) A mini drivepoint sampler for measuring pore water solute concentrations in the hyporheic zone of sand-bottom streams. Limnol Oceanogr 42:1378–1383
Duff JH, Jackman AP, Triska FJ, Sheibley RW, Avanzino RJ (2007) Nitrate retention in riparian ground water at natural and elevated nitrate levels in North Central Minnesota. J Environ Qual 36:343–353
Duff JH, Tesoriero AJ, Richardson WB, Strauss EA, Munn MD (2008) Whole-stream response to nitrate loading in three streams draining agricultural landscapes. J Environ Qual 37:1133–1144. doi:10.2134/jeq2007.0187
Fellman JB, Hood E, Spencer RGM (2010) Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: a review. Limnol Oceanogr 55:2452–2462. doi:10.4319/lo.2010.55.6.2452
Fenchel T, Blackburn TH (1979) Bacteria and mineral cycling. Academic Press
Fennel K et al (2009) Modeling denitrification in aquatic sediments. Biogeochemistry 93:159–178. doi:10.1007/s10533-008-9270-z
Fery-Forgues S, Lavabre D (1999) Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J Chem Educ 76:1260. doi:10.1021/ed076p1260
Fulweiler RW, Nixon SW, Buckley BA, Granger SL (2007) Reversal of net dinitrogen gas flux in coastal marine sediments. Nature 448:180–182. doi:10.1038/nature05963
Galloway JN et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Greenan CM, Moorman TB, Kaspar TC, Parkin TB, Jaynes DB (2006) Comparing carbon substrates for denitrification of subsurface drainage water. J Environ Qual 35:824–829. doi:10.2134/jeq2005.0247
Groffman PM, Holland EA, Myrold DD, Robertson GP, Zou X (1999) Denitrification. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard methods for long-term ecological research. Oxford University Press, New York, pp 272–288
Groffman PM et al (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Heffernan JB et al (2010) Hydrologic and biotic influences on nitrate removal in a subtropical spring-fed river. Limnol Oceanogr 55:249–263. doi:10.4319/lo.2010.55.1.0249
Hernes PJ, Bergamaschi BA, Eckard RS, Spencer RGM (2009) Fluorescence-based proxies for lignin in freshwater dissolved organic matter. J Geophys Res 114:G00F03
Heppell CM, Heathwaite AL, Binley A, Byrne P, Ullah S, Lansdown K, Keenan P, Trimmer M, Zhang H (2013) Interpreting spatial patterns in redox and coupled water–nitrogen fluxes in the streambed of a gaining river reach. Biogeochemistry. doi:10.1007/s10533-013-9895-4
Hill AR, Devito KJ, Campagnolo S, Sanmugadas K (2000) Subsurface denitrification in a forest riparian zone: the interactions between hydrology and supplies of nitrate and organic carbon. Biogeochemistry 51:193–223
Hobbie SE (1992) Effects of plant-species on nutrient cycling. Trends Ecol Evol 7:336–339. doi:10.1016/0169-5347(92)90126-V
Ishii SKL, Boyer TH (2012) Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: acritical review. Environ Sci Technol 46:2006–2017. doi:10.1021/es2043504
Jones JB, Schade JD, Fisher SG, Grimm NB (2007) Organic matter dynamics in Sycamore Creek, a desert stream in Arizona, USA. In: Webster JR, Meyer JL (eds) Stream organic matter budgets. J North Am Benthol Soc 16:78–82
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennet GE, Cornwell JC (1994) Membrane inlet mass spectrometer for high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66:4166–4170
Kaushik NK, Hynes HBN (1971) The fate of dead leaves that fall into streams. Arch Hydrobiol 68:465–515
Lovett GM, Weathers KC, Arthur MA, Schultz JC (2004) Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308
MATLAB version 8.0.0.783 (2012) Natick, Massachusetts: The MathWorks Inc
Medeiros AO, Pascoal C, Graca MAS (2009) Diversity and activity of aquatic fungi under low oxygen conditions. Freshw Biol 54:142–149. doi:10.1111/j.1365-2427.2008.02101.x
Mehring AS, Maret TJ (2011) Red maple dominance enhances fungal and shredder growth and litter processing in temporary ponds. Limnol Oceanogr 56:1106–1114. doi:10.4319/lo.2011.56.3.1106
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Metzler GM, Smock LA (1990) Storage and dynamics of subsurface detritus in a sand- bottomed stream. Can J Fish Aquat Sci 47:588–594
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174. doi:10.1890/0012-9615(2006)076[0151:ATMOLD]2.0.CO;2
Mulholland PJ et al (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452:202–205. doi:10.1038/nature06686
Nykvist N (1962) Leaching and decomposition of litter V. Experiments on Leaf Litter of Alnus glutinosa Fagus silvatica and Quercus robur. Oikos 13:232–248. doi:10.2307/3565087
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36:742–746. doi:10.1021/es0155276
Pfenning KS, McMahon PB (1996) Effect of nitrate, organic carbon, and temperature on potential denitrification rates in nitrate-rich riverbed sediments. J Hydrol 187:283–295
Puckett LJ, Zamora C, Essaid H, Wilson JT, Johnson HM, Brayton MJ, Vogel JR (2008) Transport and fate of nitrate at the ground-water/surface-water interface. J Environ Qual 37:1034–1050. doi:10.2134/jeq2006.0550
Richardson JS (1992) Coarse particulate detritus dynamics in small, montane streams in southwestern British Columbia. Can J Fish Aquat Sci 49:337–346. doi:10.1139/f92-038
Richardson WB, Strauss EA, Bartsch LA, Monroe EM, Cavanaugh JC, Vingum L, Soballe DM (2004) Denitrification in the Upper Mississippi River: rates, controls, and contributions to nitrate flux. Can J Fish Aquat Sci 61:1102–1112
Sanei H, Outridge PM, Dallimore A, Hamilton PB (2012) Mercury-organic matter relationships in pre-pollution sediments of thermokarst lakes from the Mackenzie River Delta, Canada: the role of depositional environment. Biogeochemistry 107:149–164. doi:10.1007/s10533-010-9543-1
Schipper LA, Vojvodic-Vukovic M (1998) Nitrate removal from groundwater using a denitrification wall amended with sawdust: field trial. J Environ Qual 27:664–668
Seitzinger S et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090. doi:10.1890/1051-0761(2006)016[2064:DALAWA]2.0CO;2
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24. doi:10.1023/A:1016541114786
Sobczak WV, Findlay S, Dye S (2003) Relationships between DOC bioavailability and nitrate removal in an upland stream: an experimental approach. 62:309–327. doi:10.1023/A:1021192631423
Solarzano L (1969) Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14:799–801
Starr RC, Gillham RW (1993) Denitrification and organic carbon availability in two aquifers. Groundwater 31:934–947. doi:10.1111/j.1745-6584.1993.tb00867.x
Stedmon CA (2000) Optical properties and signatures of chromophoric dissolved organic matter (CDOM) in Danish coastal waters. Estuar Coast Shelf Sci 51:267–278. doi:10.1006/ecss.2000.0645
Stedmon CA, Bro R (2008) Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol Oceanogr Methods 6:1–6
Stedmon CA, Markager S (2005) Resolving the variability of dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol Oceanogr 50:686–697. doi:10.4319/lo.2005.50.2.0686
Stelzer RS, Bartsch LA (2012) Nitrate removal in deep sediments of a nitrogen-rich river network: a test of a conceptual model. J Geophys Res Biogeosci 117:G02027. doi:10.1029/2012JG001990
Stelzer RS, Bartsch LA, Richardson WB, Strauss EA (2011a) The dark side of the hyporheic zone: depth profiles of nitrogen and its processing in stream sediments. Freshw Biol 56:2021–2033. doi:10.1111/j.1365-2427.2011.02632.x
Stelzer RS, Drover DR, Eggert SL, Muldoon MA (2011b) Nitrate retention in a sand plains stream and the importance of groundwater discharge. Biogeochemistry 103:91–107. doi:10.1007/s10533-010-9449-y
Stelzer RS, Scott JT, Bartsch LA (2015) Buried particulate organic carbon stimulates denitrification and nitrate retention in stream sediments at the groundwater-surface water interface. Freshw Sci (in press)
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Strauss EA, Lamberti GA (2002) Microbial decomposition of leaf leachates and effect of organic carbon quality on nitrification rates in stream sediments. Freshw Biol 47:65–74
Taylor PG, Townsend AR (2010) Stoichiometric control of organic carbon-nitrate relationships from soils to the sea. Nature 464:1178–1181. doi:10.1038/nature08985
Teodoru CR, del Giorgio PA, Prairie YT, St-Pierre A (2013) Depositional fluxes and sources of particulate carbon and nitrogen in natural lakes and a young boreal reservoir in Northern Quebec. Biogeochemistry 113:323–339. doi:10.1007/s10533-012-9760-x
Tiedje JM (1982) Denitrification. In: Methods of soil analysis, part 2. chemical and microbiological properties-agronomy monograph no. 9 (2nd edn). ASA-SSSA, Madison, WI, pp. 1011–1026
van Oevelen D, Middelburg JJ, Soetaert K, Moodley L (2006) The fate of bacterial carbon in an intertidal sediment: modeling an in situ isotope tracer experiment. Limnol Oceanogr 51:1302–1314
von Elert E, Martin-Creuzburg D, Le Coz JR (2003) Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc R Soc B Biol Sci 270:1209–1214. doi:10.1098/rspb.20033.2357
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Ann R Ecol Syst 17:567–594
Wilson HF, Xenopoulos MA (2008) Ecosystem and seasonal control of stream dissolved organic carbon along a gradient of land use. Ecosystems 11:555–568. doi:10.1007/s10021-008-9142-3
Yoshimura C, Fujii M, Omura T, Tockner K (2010) Instream release of dissolved organic matter from coarse and fine particulate organic matter of different origins. Biogeochemistry 100:151–165. doi:10.1007/s10533-010-9412-y
Zarnetske JP, Haggerty R, Wondzell SM, Baker MA (2011) Labile dissolved organic carbon supply limits hyporheic denitrification. J Geophys Res Biogeosci 116:G04036. doi:10.1029/2011JG001730
Acknowledgments
We thank Courtney Heling, Mike Louison, Alyssa McCumber and Erin Grantz for technical assistance. Will Cook at Duke University conducted the CN analysis and Jeff Merriam at the United States Forest Service performed the DOC and DON analysis. We thank two anonymous reviewers for their comments on the manuscript. This research was supported by a grant from the University of Wisconsin Water Resources Institute through the Wisconsin Groundwater Coordinating Council. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jennifer Leah Tank.
Rights and permissions
About this article
Cite this article
Stelzer, R.S., Thad Scott, J., Bartsch, L.A. et al. Particulate organic matter quality influences nitrate retention and denitrification in stream sediments: evidence from a carbon burial experiment. Biogeochemistry 119, 387–402 (2014). https://doi.org/10.1007/s10533-014-9975-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-9975-0