Abstract
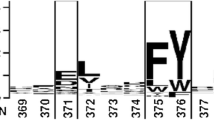
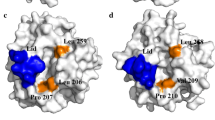
Error-prone PCR was used to create more thermoactive and/or thermostable variants of thermoalkalophilic lipases. A variant of the α6 helix (lid domain), with an 189E to V substitution at residue 189, lost its thermostability but exhibited higher activity than that of its wild-type predecessor (r03Lip). Site-saturation mutagenesis was used to explore the sequence-function relationship. Five other mutants also lost thermostability (20–40%) but exhibited enhanced thermoactivity (6.3–79-fold). The mutant E189I showed the highest activity retaining 50% activity after maintaining it at 65°C for 24 h. In comparison to r03Lip, the mutant E189I had a higher affinity for p-nitrophenyl palmitate and p-nitrophenyl stearate (61 and 56% decreased Km) and catalytic efficiency (42-fold and 18-fold increased kcat/Km). The mutant lipase retained its tolerance to n-hexane, but had an improved transesterification activity. The results suggest that residue Glu189 plays a significant role in the thermostability and activity of this thermoalkalophilic lipase.


Similar content being viewed by others
References
Ahmad S, Kamal MZ, Sankaranarayanan R, Rao NM (2008) Thermostable Bacillus subtilis lipases: in vitro evolution and structural insight. J Mol Biol 381:324–340
Carrasco-López C, Godoy C, de Las Rivas B, Fernández-Lorente G, Palomo JM, Guisán JM, Fernández-Lafuente R, Martínez-Ripoll M, Hermoso JA (2009) Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements. J Biol Chem 284:4365–4372
Gao B, Xu T, Lin JP, Zhang LJ, Su EZ, Jiangand ZG, Wei DZ (2011) Improving the catalytic activity of lipase LipK107 from Proteus sp. by site-directed mutagenesis in the lid domain based on computer simulation. J Mol Catal B 68:286–291
Jeong ST, Kim HK, Kim SJ, Chi SW, Pan JG, Oh TK, Ryu SE (2002) Novel zinc-binding center and a temperature switch in the Bacillus stearothermophilus L1 lipase. J Biol Chem 277:17041–17047
Khurana J, Singh R, Kaur J (2011) Engineering of Bacillus lipase by directed evolution for enhanced thermal stability: effect of isoleucine to threonine mutation at protein surface. Mol Biol Rep. doi:10.1007/s11033-010-9954-z
Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T (2009) The SWISS-MODEL Repository and associated resources. Nucleic Acids Res 37:D387–D392
Kleiger G, Grothe R, Mallick P, Eisengber D (2002) GXXXG and AXXXA: common α-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry 41:5990–5997
Kordel M, Hofmann B, Schomburg D, Schmid RD (1991) Extracellular lipase of Pseudomonas sp. strain ATCC 21808, purification, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol 173:4836–4841
Marqusee S, Baldwin RL (1987) Helix stabilization by Glu−···Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci USA 84:8898–8902
Matsumura H, Yamamoto T, Leow TC, Mori T, Salleh AB, Basri M, Inoue T, Kai Y, Rahman RN (2008) Novel cation-pi interaction revealed by crystal structure of thermoalkalophilic lipase. Proteins 70:592–598
Santarossa G, Lafranconi PG, Alquati C, DeGioia L, Alberghina L, Fantucci P, Lotti M (2005) Mutations in the “lid” region affect chain length specificity and thermostability of a Pseudomonas fragi lipase. FEBS Lett 579:2383–2386
Teng Y, Xu Y (2007) A modified para-nitrophenyl palmitate assay for lipase synthetic activity determination in organic solvent. Anal Biochem 363:297–299
Tyndall JD, Sinchaikul S, Fothergill-Gilmore LA, Taylor P, Walkinshaw MD (2002) Crystal structure of a thermostable lipase from Bacillus stearothermophilus P1. J Mol Biol 323:859–869
Vinther JM, Kristensen SM, Led JJ (2011) Enhanced stability of a protein with increasing temperature. J Am Chem Soc 133:271–278
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shih, TW., Pan, TM. Substitution of Asp189 residue alters the activity and thermostability of Geobacillus sp. NTU 03 lipase. Biotechnol Lett 33, 1841–1846 (2011). https://doi.org/10.1007/s10529-011-0635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0635-3