Abstract
Pollution and diseases such as the coronavirus pandemic (COVID-19) are major issues that may be solved partly by nanotechnology. Here we review the synthesis of ZrO2 nanoparticles and their nanocomposites using compounds from bacteria, fungi, microalgae, and plants. For instance, bacteria, microalgae, and fungi secret bioactive metabolites such as fucoidans, digestive enzymes, and proteins, while plant tissues are rich in reducing sugars, polyphenols, flavonoids, saponins, and amino acids. These compounds allow reducing, cap**, chelating, and stabilizing during the transformation of Zr4+ into ZrO2 nanoparticles. Green ZrO2 nanoparticles display unique properties such as a nanoscale size of 5–50 nm, diverse morphologies, e.g. nanospheres, nanorods and nanochains, and wide bandgap energy of 3.7–5.5 eV. Their high stability and biocompatibility are suitable biomedical and environmental applications, such as pathogen and cancer inactivation, and pollutant removal. Emerging applications of green ZrO2-based nanocomposites include water treatment, catalytic reduction, nanoelectronic devices, and anti-biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution and climate change are presenting as two of the most globally critical issues in the context of the coronavirus pandemic (COVID-19) and circular economy (Muhammad et al. 2020; Ufnalska and Lichtfouse 2021). The advent of nanoscience and nanotechnology can solve these problems; thereby, holding the key to economic recovery, and environmental mitigation (Weiss et al. 2020; Tang et al. 2021). Nanomaterials play a central role in nanotechnology, raising the demands for sustainable development (Srivastava et al. 2021). The chemical synthesis of nanomaterials chiefly does not satisfy the strict requirements for this trend, and also mismatches with the principles of green chemistry. Currently, the green approaches for the synthesis of nanomaterials are of remarkable significance due to their eco-friendliness as well as cost-effectiveness (Tran et al. 2020a). This also pays the way for multiple applications of nanomaterials in the environmental mitigation, biomedical, and catalysis fields.
Zirconium (Zr) is classified as a transition metal element (d-block) in the titanium family (group IV) with the atomic number 40. Zr does not basically capture neutrons, offering a potential metallic cladding for the fuel rods in the nuclear reactors (Cazado et al. 2021). Zirconium dioxide (ZrO2) or zirconia is one of the highly stable oxides, created by thermalizing zirconium compounds (Hassan and Jalil 2022). Depending on the various synthesis routes, ZrO2 can present in the crystalline phases involving monoclinic, tetragonal, and cubic (Zhang et al. 2018). Bulk ZrO2 has a wide bandgap energy, typically ranging from 5.0 to 7.0 eV. Because of the ultrahigh stability and very low toxicity, ZrO2 has exhibited an intensive range of practical technologies for heat-resistant ceramic superalloys (Wang et al. 2021), dental restorations (Chen et al. 2021), fuel cells (Rambabu et al. 2020), and heterogeneous catalysis (Jiang et al. 2020). Such promising utilizations make ZrO2 an ideal nanomaterial, promoting the green strategy for synthesizing ZrO2 nanoparticles.
In general, there are two major approaches to synthesize the ZrO2 nanoparticles, involving top-down and bottom-up (Jadoun et al. 2021). The former implies the conversion of bulk material into thinner crystallites by the physical route. This means that it needs the participation of enormous mechanical energy sources such as milling, and ionic sputtering (Shrimal et al. 2020). As a result, top-down strategy brings many inevitable drawbacks of causing secondary impressions or intermediates, altering the physicochemical property and surface chemistry of as-synthesized nanoparticles (Indiarto et al. 2021). More importantly, nano-sized particles are mostly unattainable by top-down approach. Meanwhile, the latter implies the formation of particles by creating building blocks from ultra-small particles such as atoms or molecules, and then assembling them together. By this way, the nanostructured particles can be intentionally attainable under the control of fabrication conditions (Rana et al. 2020). The bottom-up strategy involves the physical (e.g., vapor decomposition, plasma irradiation, and ultrasonication), chemical (e.g., sol–gel, co-precipitation, chemical reduction, and hydrothermal), and biological (e.g., plant, fungi, algae, and bacteria) methods. However, the physical bottom-up method requires a high investment cost for operating instruments and consumes a large amount of heating or electric energies (Bolade et al. 2020). The chemical bottom-up method necessarily adopts toxic chemicals for its protocols, resulting in adverse impacts on the environment. Therefore, it may be greatly difficult for physical and chemical methods to attain a critical green strategy.
The biological bottom-up method is the most adequate for the green synthesis of ZrO2 nanoparticles because it offers an effective, tunable, and eco-friendly approach (Shafey 2020). The biogenic synthesis uses low-cost and locally available sources such as plants or other biocompatible sources such as fungi, algae, and bacteria for ZrO2 fabrication (Rana et al. 2020; Nguyen et al. 2021d). The biomolecules extracted from the biological sources play a vital role as extremely efficient bioreducing, biocap**, and biostabilizing agents, bringing excellent ZrO2 production yields (Bandeira et al. 2020). Such biological substrates can safely replace almost highly expensive and toxic chemicals or energy-consuming physical instruments (Agarwal et al. 2017). Biological method for synthesis of ZrO2 nanoparticles also match well with principle of sustainable and green chemistry (Yadi et al. 2018). Because of diminishing the potential risks from chemical and physical methods, generating no hazardous intermediates, and secondary pollutions, biogenic synthesis of ZrO2 nanoparticles can be therefore considered as the green synthesis (Jadoun et al. 2021).
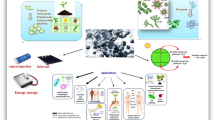
To our knowledge, there is only a limited number of relevant works (about twenty research articles) reported the green synthesis of ZrO2 nanoparticles using various biological sources such as bacteria, fungi, and plant extract. Moreover, there is currently no comprehensive review on the systematic evaluations from the green synthesis to the applications of ZrO2 nanoparticles and their nanocomposites. Therefore, the main objectives of this study are to discuss the current findings on green ZrO2 preparation techniques and highlight their performance in the biomedical field and environmental remediation. Specifically, this review has the following organization: (i) green synthesis methods for ZrO2 nanoparticles; (ii) structural characterizations of green ZrO2 nanoparticles; (iii) biomedical, adsorption, catalytic, and other applications of green ZrO2 nanoparticles; and (iv) emerging applications of green ZrO2-based nanocomposites (Fig. 1). We briefly elucidate some challenging and prospective aspects of green ZrO2 nanocomposites for expanding their potential applications.
Green synthesis of ZrO2 nanoparticles and their applications in biomedical, adsorption, catalysis, and nanocomposite fabrication. The plant tissues including flowers, fruits, seeds, leaves, roots, etc. possess many phytochemicals such as polyphenols, saponins, quercetin, and gallic acid. Microorganisms including bacteria, fungi, algae, etc. can secrete biomolecules, metabolites, enzymes, and proteins. These phytochemicals and biomolecules participate in the green synthesis of ZrO2
Green synthesis of ZrO2 nanoparticles
Bacteria
Bacteria are among the fastest-growing microorganisms under mild cultivation conditions in temperature, pressure, and pH (Ram et al. 2020). They are therefore the favorable biofactories for the synthesis of ZrO2 nanoparticles. During the metabolism activities, bacteria secrete some enzymes and proteins extracellularly or intracellularly, which possibly aid the bioreduction, biocap**, and biostabilization of zirconium ions (Fariq et al. 2017). Underlying mechanism of forming ZrO2 nanoparticles by bacterial communities endures further complex steps including (i) biosorption and (ii) bioreduction (Saravanan et al. 2021). The former step initiates with trap** zirconium ions on the bacterial cell surface through the physical and chemical interactions (e.g., electrostatic, hydrogen bonding, ionic exchanging, and chelating) (Gahlawat and Choudhury 2019). Specifically, the secretion of macromolecules such as proteins, polysaccharides containing many negatively charged functional groups enriches the surface chemistry of bacterial cell wall (Patil and Kim 2018). This creates the attraction of Zr4+ ions towards bacterial cell surface. The latter step takes main responsibility for reducing Zr4+ ions into ZrO2 nanoparticles. Bioactive compounds such as acid amines, proteins, etc. in the extracellular or intracellular environment can participate in the formation and stabilization of ZrO2 nanoparticles (Narayanan and Sakthivel 2010).
Suriyaraj et al. (2019) reported the one-step biofabrication of ZrO2 nanoparticles using extremophilic Acinetobacter sp. bacterial community. This green source was firstly cultivated in glucose solution, and added by 100 mM ZrOCl2·8H2O for incubation for 12 h. Interestingly, highly crystalline ZrO2 nanomaterial was facilely precipitated under room-temperature condition (37 °C). Moreover, this study found biocompatibility and ineligible cytotoxicity with mouse fibroblast cells. The fluorescein staining was carried out to confirm the major role of extracellular proteins released from Acinetobacter sp. for biosynthesis of ZrO2. Very recently, Ahmed et al. (2021) successfully synthesized zirconia nanoparticles using Enterobacter sp. strain. Notably, the bacterial strain was facilely isolated from the paddy soils, and then, incubated at room temperature. After the bacterial purification, 5 mM ZrOCl2·8H2O was addedto the supernatant under agitating incubation for 24 h. Visible precipitation was finally formed at the bottom of the flask. It was proposed that nitrate reductase enzyme was in charge of reducing Zr4+ ions into zirconia and protein secreted by Enterobacter sp. acts as a cap** agent (Fig. 2). As-biosynthesized ZrO2 nanoparticles revealed the promising antifungal activity with 75.5% inhibition against Pestalotiopsis Versicolor disease pathogen. These publications have demonstrated the great potentials and biocompatibility of the bacterially synthesized ZrO2 nanoparticles.
Extracellularly biosynthesized mechanism of zirconia nanoparticles using Enterobacter sp. strain. Bacteria extracellularly secrete some specific enzymes such as reductase, which take responsibility for enzymatic bioreduction of Zr4+ ions. The underlying mechanism is attributable to the redox of nicotinamide adenine dinucleotide (NAD+/NADH), which supplies electrons to the reduction of Zr4+ ions during nucleation. Cap** proteins can also be released into the extracellular environment and aid the biocap** and biostabilization of zirconia nanoparticles. Reprinted with the permission of Elsevier from reference (Ahmed et al. 2021). Abbreviations: ZrONPs, zirconia nanoparticles; NAD+, an oxidized form of nicotinamide adenine dinucleotide; NADH, a reduced form of nicotinamide adenine dinucleotide
Fungi
Fungi include the microbial organisms that absorb nutrients by decomposing organic matters, without the photosynthesis pathways (Chu et al. 2021). They play the principal role in nutrient cycling and exchanging as well as mitigation of metals-contaminated soils (Riaz et al. 2021). During these processes, fungal communities secrete digestive enzymes into their foods to acquire the nutrients essential for growth (Ferreira et al. 2020). Taking advantage of such features, fungi can be ideal biotemplates for the synthesis of ZrO2 nanoparticles. Basically, the mechanism for green synthesis of ZrO2 nanoparticles using fungal species is closely similar to that using bacterial species. However, the utilization of fungi for ZrO2 production offers many advantageous points (Narayanan and Sakthivel 2010). Firstly, they can endure the harsh synthesis conditions such as flow pressure or agitation in the bioreactor, easily handling the fabrication of ZrO2 (Abinaya et al. 2021). Secondly, fungi have accelerated growth in controllable ways, i.e., through adjusting their nutrient ingredients. Ultimately, almost all fungi are better resistant to genetic or environmental mutations, facilitating the culturing process (Bansal et al. 2011). These features of fungal species are more favorable for biosynthesis of ZrO2 than other biological synthesis methods such as bacteria and plants.
Ghomi et al. (2019) reported that the formation of ZrO2 by extracellular secretion of Penicillium fungal species including P. aculeatum, P. notatum, and P. purpurogenome. Under the optimum conditions at pH 9, and 1.5 mM Zr4+, the solution color changed from pale yellow into deep yellow after seven-day incubation, affirming the generation of colloidal ZrO2. In terms of the ZrO2 biofabrication mechanism, the enzymes presenting in the fungal supernatants may aid to transform Zr4+ ions into ZrO2 nanoparticles. Bansal et al. (2004) cultured another fungal species named Fusarium oxysporum for the successful biosynthesis of ZrO2 nanoparticles from the ZrF62− source at room temperature. To gain insight into the major role of proteins secreted from this fungus for the extracellular hydrolysis of ZrF62−, the resulting filtrates were lyophilized to test for hydrolytic activity. The findings confirmed the cationic nature of proteins to bind easily to ZrF62− anions, which could not be found in other fungal species (e.g., C. lunata, C. gloeosporioides, Phomopsis sp., A. niger). More importantly, ZrF62− anions showed a non-toxicity to Fusarium oxysporum, opening the enormous prospects for large-scale biosynthesis of ZrO2 nanoparticles. To sum up, the highlighted eco-friendly, biocompatible, and energy-conserving advantages of the fungal biosynthesis of ZrO2 nanoparticles are superior to chemical methods, and hence, should not be overlooked.
Algae
Great difference from fungal species, algae are a group of dominantly aquatic, eukaryotic, and autotrophic organisms that acquires their nutrients from the photosynthetic processes. Algae use chlorophyll for their primary photosynthesis; therefore, they are sometimes considered as terrestrial plants. Some algal species such as brown algae, or seaweeds excrete fucoidans from their cell walls (Filote et al. 2021). Fucoidans refer to multifunctional polysaccharides containing a substantial amount of fucose and sulfated esters, exhibiting many bioactivities involving antiviral, antioxidant, and anticoagulant (Ponce and Stortz 2020). These biomolecules can therefore act as ideal bioreducers for the green synthesis of ZrO2. Indeed, Kumaresan et al. (2018) successfully bioprepared tetragonally nanostructured ZrO2 from the aqueous extract of seaweed Sargassum wightii. The biosynthesis procedure was tunable with the grind of ZrO(NO3)2·H2O with Sargassum wightii extract within 20 min. ZrO2 nanoparticles could be formed after the calcination at 400 °C. This nanomaterial showed the spherical morphology with the particle size of 5 nm and was utilized for investigating several potential antibacterial activities against gram-positive and gram negative-bacteria. The researchers demonstrated that large surface area and nanosize (4.8 nm) of ZrO2 obtained from this green strategy contributed considerably to enhancing growth inhibitory effects against antibacterial pathogens. Although many works reported the use of algae for biosynthesizing various nanoparticles, there is still an exception for ZrO2. It is also necessary to elucidate the underlying mechanisms for the formation of ZrO2 nanoparticles using algal species.
Plants
Plants are the most prevalent, locally available, highly cost-effective resource for biosynthesis of ZrO2 nanoparticles (Yadi et al. 2018). Compared with other green materials such as bacteria, fungi, and algae, the use of plants as biotemplates attains many notable benefits (Saravanan et al. 2021). Firstly, there are less risks to health than microbial ZrO2 synthesis strategies. Almost plants are substantially more benign than bacteria or fungi. Some microbial species can even secrete toxins or metabolites during cultivation (Yang and Chiu 2017; Xu et al. 2020). Secondly, the botanical synthesis of ZrO2 nanoparticles is considerably accelerated in comparison with microbial routes. The bioreduction of Zr4+ by biomolecules from plant extract occurs within a few minutes to several hours, while microbial communities mostly prolong these bioprocesses by many days under ambient incubation (Vijayaraghavan and Ashokkumar 2017). This results in the higher kinetic of overall ZrO2 production process. Thirdly, the synthesis of ZrO2 nanomaterial by plant extracts is controllable and easy to manipulate. Diverse parts of plants such as leaves, barks, roots, flowers, etc. can be effortlessly collected for biosynthesis of ZrO2 nanoparticles. The green solvents for extracting phytochemical compounds from plant tissues are mainly water and ethanol. Meanwhile, the microbial culture needs to be carried out under strict conditions to avoid the generation of colonies, unfavorable to ZrO2 fabrication.
Phytochemicals involving reducing sugars, polyphenols, flavonoids, saponins, and amino acids are inherent in the plant tissues such as flowers, leaves, and roots (Fig. 3a). These compounds are known as strong antioxidants or bioreductants, which can play pivotal roles as biocap**, biochelating, bioreducing, and biostabilizing agents for the synthesis of ZrO2 nanoparticles (Fig. 3b). The phytochemicals participate in the formation and stabilization of octahedral complex of Zr2+–phytochemicals. The presence of phytochemicals also aids to hamper the aggregation of ZrO2 nanoparticles during thermal calcination. This may boost the surface area of ZrO2 nanoparticles, which are conducive to their catalytic and adsorption properties. Consequently, the phytochemicals in plant extract can exhibit a range of important functionalities including replacing hazardous, expensive chemical antioxidants or reductants, and shortening the number of complicated synthesis steps.
Phytochemicals in plant extracts (a), and proposed mechanism for the biosynthesis of ZrO2 nanoparticles using these phytochemicals (b). Phytochemicals act as bioreductants to convert Zr4+ into octahedral complex [Zr(H2O)6]2+. This enhances the stability and hinders the clustering of zirconium complexation during chelation with phytochemicals Zr2+–phytochemicals. The calcination of Zr2+–phytochemicals complex produces ZrO2 nanoparticles in tandem with the release of CO2, H2O, N2, and many decomposed products
The general botanical biosynthesis of ZrO2 nanoparticles initiates with the collection and pretreatment of plant tissues to eliminate the irrelevant parts (Table 1). The procedure is followed by extracting phytochemicals using several common kinds of solvents such as water, ethanol, or hexane from cell walls (Saraswathi and Santhakumar 2017; Goyal et al. 2021; Alagarsamy et al. 2022). Water is a prevalently used, and highly polar extractor which recovers the aqueously soluble phytochemical compounds such as gallic acid, polyphenols, glucose, etc. while ethanol exhibits a poorer polarity than water, but higher than hexane. There are two major steps including complexation and calcination to reach the final ZrO2 products. The former is carried out under the incorporation of plant extract into zirconium source, mainly ZrOCl2·8H2O. The proper temperature and time for forming Zr2+–phytochemicals are 55–75 °C, and 1–4 h, respectively. This step can also be assisted by microwave irradiation (900 W) to accelerate the complexation rate up to 15 min (Shinde et al. 2018), or by alkaline addition (Joshi et al. 2021). The Zr2+–phytochemicals complex is totally separated by centrifugation or paper filtration (Gowri et al. 2014). The latter experiences the thermal calcination at high temperature (500–800 °C) for 2–4 h to convert this complex into ZrO2 nanoparticles.
Properties of green ZrO2 nanoparticles
Crystallinity
Bulk ZrO2 nanoparticles exist in three common crystalline phases involving monoclinic, tetragonal, and cubic. Biologically synthesized ZrO2 nanoparticles can also attain such crystalline phases based on X-Ray diffraction analysis (Table 2). Specifically, Davar et al. (2018) reported a cubic phase of ZrO2 nanoparticles bioproduced from Salvia Rosmarinus plant extract with the reflecting planes at (111), (200), (220), (311), (222), and (400). Another study indicated the monoclinic phase of ZrO2 synthesized from Helianthus annuus plant extract (Goyal et al. 2021). However, tetragonal phase is the most prevalent single-crystal phase of green ZrO2 nanoparticles. Green ZrO2 nanoparticles can also have multiphase crystallinity, which includes at least two phases (Sathishkumar et al. 2013; Debnath et al. 2020; Isacfranklin et al. 2020). The presence of crystal phase in green ZrO2 possibly affects their electronic properties, optical band gap, and surface energy, and stability. It is therefore essential for further studies to clarify the role of synthesis conditions, e.g., the ratio between zirconium and plant extract in the formation of ZrO2 phase.
Optical bandgap
The bandgap energy of a semiconductor reflects the energy disparity between the top of valence band and the bottom of conduction band, or the minimum energy to excite an electron from the valence band to the conduction band (Makuła et al. 2018). It can be determined from diffuse reflectance spectra. While the bulk ZrO2 nanoparticles have a wide bandgap energy (~ 5.0 eV), green ZrO2 nanoparticles exhibit values ranging from 3.7 to 5.5 eV (Table 2). Indeed, Goyal et al. (2021) fabricated ZrO2 nanoparticles using methanolic extract of Helianthus annuus seeds with very narrow bandgap (3.7 eV). Al-Zaqri et al. (2021) found the same small value of ZrO2 nanoparticles biosynthesized from Wrightia tinctoria leaf extract. They also gave an assumption about more contribution from external surface defects ZrO2 nanoparticles. Meanwhile, obtaining higher bandgap energies (5.4–5.5 eV) of other green ZrO2 nanoparticles may be ascribed by quantum confinement effects (Gowri et al. 2014; Alagarsamy et al. 2022). The bandgap augments for the smaller size nanoparticles due to the deformity phenomena. Overall, biologically synthesized ZrO2 nanoparticles acquire sufficient gap energies, capable of exhibiting their excellent catalytic and biomedical activities through the formation of reactive oxygen species.
Particle size
Many physicochemical techniques such as X-Ray diffraction, dynamic light scattering, transmission electron microscopy, etc. can be used to measure the average particle size of ZrO2 nanoparticles. From the database of previous studies (Table 2), the particle size of ZrO2 nanoparticles is distributed from 5 to 150 nm, commonly less than 50 nm. Compared with chemical approaches, biological methods generally create ZrO2 nanoparticles with considerably smaller sizes (Suriyaraj et al. 2019; Ahmed et al. 2021). It can be understandable that biomolecules from the secretion of microbial sources, or phytochemicals from plant extracts lessen the assembly of ZrO2 nanoparticles (Kumaresan et al. 2018; Silva et al. 2019; Debnath et al. 2020). Small-size ZrO2 nanoparticles offer many biomedical benefits since they facilitate the penetration into cell walls, performing better antibacterial, antifungal, and anticancer activities (Khatoon et al. 2015).
Morphology
The shape of ZrO2 nanocrystals biosynthesized from biological routes can be explored by scanning/transmission electron microscopy analysis. Accordingly, green ZrO2 nanoparticles can present a wide range of morphological properties such as nanospheres (mainly), nanochains, nanorods, semi-nanospheres, nano-sized ovals, and nanoflakes (Fig. 4). Generally, the ZrO2 morphology is significantly dependent on the synthesis conditions including the ratio between zirconium source and green extract. Spherical ZrO2 can be synthesized by using microbial sources with the long incubation (1–3 days) at room temperature or by several botanical sources such as Euclea natalensis roots, Aloe vera leaves, Ficus benghalensis leaves, Moringa oleifera leaves, Helianthus annuus seeds, Tinospora cordifolia leaves, and Laurus nobilis leaves (Table 2). Meanwhile, other ZrO2 morphologies are relatively rare, mostly synthesized by plant sources. For example, Sathishkumar et al. (2013) obtained ZrO2 nano-chains by the hydrolysis of ZrF62− in the presence of Curcuma longa tuber extract. Gowri et al. (2015) also successfully biosynthesized ZrO2 nanoflakes by the hydrolysis of ZrOCl2·8H2O using aqueously soluble carbohydrates extracted from Nyctanthes arbor-tristis flower. Semi-spherical and oval ZrO2 can be formed from many plant extracts such as Salvia Rosmarinus and Lagerstroemia speciose, respectively (Saraswathi and Santhakumar 2017; Davar et al. 2018). More importantly, diverse morphologies of ZrO2 morphologies can bring many benefits for practical applications. As an example, (Isacfranklin et al. 2020) indicated that ZrO2 nanorods possess sufficient cellular uptake and toxicity, offering the prospect for biomedical application.

source 3:1 (b), and 6:1 (c). The role of plant extract is to improve the dispersion of ZrO2 nanoparticles during biosynthesis. Reproduced and adapted with the permission of Elsevier from reference (Davar et al. 2018). Transmission electron microscopy photographs of ZrO2 nanospheres (d), nano chains (e), and nanorods (f) biosynthesized from Acinetobacter sp. bacterial community (Suriyaraj et al. 2019), Curcuma longa tuber extract (Sathishkumar et al. 2013), and Nephelium lappaceum fruit extract (Isacfranklin et al. 2020), respectively. Reproduced and adapted with the permission of Elsevier from references (Sathishkumar et al. 2013; Suriyaraj et al. 2019; Isacfranklin et al. 2020)
Field emission scanning electron microscope photographies of ZrO2 nanoparticles produced at 600 °C without Rosmarinus officinalis leaf extract (a), and from Rosmarinus officinalis leaf extract with the ratio between the extract and zirconium
Surface chemistry
The surface functionalization by chemical groups aids to extend the applications of nanomaterials (Tran et al. 2021). Surface chemistry of ZrO2 nanoparticles can be identified by several physicochemical techniques such as Fourier-transform infrared spectroscopy and X-ray photoelectron spectroscopy or quantified by Boehm titrations (Nguyen et al. 2021b). The surface functional groups on ZrO2 nanoparticles may be originated from the phytochemical compounds. Considering the calcination of the complex of Zr2+–phytochemicals, almost all organic components are decomposed into volatiles or simple products such as CO2, N2, and H2O. A tiny proportion of phytochemicals may still remain due to the chelating with zirconium ions. They can be thermally modified to generate simpler biomolecules with more diverse functional groups such as carbonyl, amine, carboxylate, and alkyl (Table 2). However, hydroxyl groups are detected partly owing to H2O-adsorbed surface of ZrO2 (Kumaresan et al. 2018; Silva et al. 2019; Annu et al. 2020). The functionalization of ZrO2 surface possibly can lead to the improvement of adsorption efficiency towards many pollutants through many key interactions such as electrostatic attraction, hydrogen bond, Yoshida hydrogen bond, and π–π interaction (Suresh et al. 2015; Nguyen et al. 2021c).
Surface area
Theoretically, surface area of nanomaterials links closely to the number of active sites on the edges, resulting in a significant influence on their catalytic activity (Jiang et al. 2011). The higher surface area of ZrO2 nanoparticles can also facilitate the better favorable adsorption process. The bio compounds such as proteins, enzymes, polysaccharides, polyphenols, etc. present in the biological source extract chelating with zirconium ions can prevent the aggregation of ZrO2 nanoparticles during the thermolysis. This enhances the surface area and the number of active sites of ZrO2 nanoparticles. For example, Shinde et al. (2018) reported the surface area (88 m2/g) of ZrO2 nanoparticles biosynthesized from Ficus benghalensis leaf extract using BET (Brunauer, Emmett and Teller) analysis. It was also witnessed an enhancement of the photocatalytic activities of ZrO2 to methylene blue (91%) and methyl orange (69%) for 240 min. These results of green ZrO2 were remarkably higher than those obtained by the chemical methods such as anodization in H2O2/NH4F/ethylene glycol electrolyte (Rozana et al. 2017), decomposition of Zr(OH)4-urea complex (Sudrajat et al. 2016), polymer-assisted sol–gel synthesis (Dhandapani et al. 2016).
Stability
Compared with chemically produced nanomaterials, biologically produced ZrO2 nanoparticles not only exhibit good dispersion but also show better stability. For example, Sathishkumar et al. (2013) found the surprising stability of zirconia nanoparticles up to five months after green fabrication. This phenomenon may be elucidated by the presence of phytochemicals acting as efficient biostabilizers and biocap** agents. The stability of ZrO2 nanoparticles can be assessed by the zeta potential measurements. Basically, the zeta potential values are different with zero, normally minimum ± 30 mV, indicating the high stability of ZrO2 nanoparticles (Jameel et al. 2020). Indeed, Suriyaraj et al. (2019) demonstrated the well-dispersed, anti-clustering, and nanostructured ZrO2 suspension by the measurement of zeta potential (36.5 ± 5.5 mV). Chau et al. (2021) also measured the high zeta potential (− 32.8 mV) of ZrO2 nanoparticles biosynthesized from Laurus nobilis leaf extract by dynamic light scattering analysis, proposing the good anti-sedimentation of the nanomaterials for antimicrobial activities.
Applications of green ZrO2 nanoparticles
Biomedical applications
Biomedical compatibility of green ZrO2 nanoparticles
Bacterial and fungal species attack the immune system of humans and animals and become the major factors causing infections and even deaths (Humbal et al. 2018). Many antibiotics and antifungal pharmaceuticals have been developed over the centuries, but mankind is increasingly encountering the huge problems of antibiotic resistance (Kovalakova et al. 2020). Among the efforts to search for alternatives to antibiotics, bionanotechnology exhibits its high reliability and performance contributing proactively to the abatement of infections (Ong et al. 2018). Many metallic nanomaterials (e.g., silver nanoparticles) possess their inherent antibacterial and antifungal activities; hence, they are widely utilized in biomedical fields (Abbasi et al. 2014). However, the lack of biocompatibility, biostability, and effectivity may restrain the biomedical application of these nanomaterials. Green ZrO2 nanoparticles biosynthesized from environmentally friendly botanical and microbial sources show unique properties, adequate for fabricating biomedical devices. Based on the published literature, this section will discuss some potential activities of green ZrO2 nanoparticles.
Antibacterial activity
The plasma membrane of bacteria is mostly constituted of proteins (e.g., peptidoglycan macromolecules) that charge negatively (Li et al. 2019). ZrO2 nanoparticles charge positively on their surface, leading to electrostatic interactions with bacterial membranes (**, biochelating, and biostabilizing process for the transformation into composites. For example, Vivekanandhan et al. (2015) functionalized the surface of ZrO2 nanocrystallites with silver nanoparticles using Anacardium occidentale leaf extract (Fig. 6). The bio compounds from this extract might participate in the bioreduction of Ag+ into Ag and nucleation of Ag nanospheres (5–20 nm) on ZrO2 surface. The authors also reported the inhibition of the deformation of Ag/ZrO2 nanocomposites in the larger presence of leaf extract. This phenomenon may be attributable to swift bioreduction of Ag+ ions before their dispersion on the surface of ZrO2. Therefore, a sufficient amount of plant extract is important to obtain the structure of ZrO2-based nanocomposites. Table 5 summaries several potential applications of green ZrO2-based nanocomposites.
The bioreduction of Ag+ into Ag and nucleation of on ZrO2 surface for the biosynthesis of green Ag/ZrO2 nanocomposites using Anacardium occidentale leaf extract (a); the photograph of as-synthesized green ZrO2 and Ag/ZrO2 nanocomposites (b); scanning electron microscopy (c, d) and transmission electron microscopy (e, f) microphotographies of green Ag/ZrO2 nanocomposites. The presence of leaf extract efficiently leads to the good dispersion and high biostabilization of Ag nanospheres (5–20 nm) on the surface of ZrO2. However, larger addition of leaf extract inhibits the formation of Ag/ZrO2 nanocomposites. Reproduced and adapted with the permission of Elsevier from reference (Vivekanandhan et al. 2015). Abbreviations: AgNP, silver nanoparticles
Because ZrO2 nanoparticles exhibit a wide bandgap (~ 5.0 eV), the absorption of visible light is difficult, hampering their photocatalytic and nanoelectronic applications. The modification of ZrO2 nanoparticles aims to suffer this major drawback. For example, Yadav et al. (2021) inserted an amount of transition metal nickel (Ni) into ZrO2 nanoparticles using rubber latex extract to obtain monoclinic, tetragonal, and cubic Ni-doped ZrO2 polycrystalline. The band gaps were very narrowed between 2.4 and 2.75 eV, making Ni-doped ZrO2 as potential nanoelectronic devices. Rasheed et al. (2020) reported the utilization of Daphne alpine leaf extract to synthesize green V2O5/ZrO2 nanocomposite. They not only observed the increase in surface area (214 m2/g) but also found the improvement of bandgap energy (3.93 eV) and thermal stability (1000 °C). It was suggested that V2O5 efficiently enhanced the electron separation, resulting in good photocatalytic activities against orange (76.9%) and picloram (86%) of green V2O5/ZrO2.
Do** other transition metals (Sm, Cu, Ag) significantly changes the electronic nature and crystalline phases of the nanocomposites, leading to the enhancement of their stability, recyclability, and photocatalytic activities. Indeed, Gurushantha et al. (2016) fabricated the green ZrO2 with samarium dopant (Sm3+/ZrO2+ = 3–11 mol.%) using Leucas Aspera extract. Sm/ZrO2 composites with the do** of 11% dopant obtained highly symmetric nanocubics, and the highest photocatalytic degradation of acid green with six consecutive recycles in the presence of solar energy. This suggested that the Sm/ZrO2 composites are highly stable and reusable. Hamad et al. (2019) demonstrated the presence of 7-hydroxy-4´-methoxy-isoflavon from Commelina diffusa leaves reduced the agglomeration of Cu/ZrO2 nanoparticles (18–25 nm) with good stable ability during the post-synthesis. Moreover, the green Cu/ZrO2 nanoparticles catalyzed the reduction and degradation of 2,4-dinitrophenylhydrazine, and organic dyes including congo red, nigrosin, methyl orange with ultrafast reaction times (1–150 s). In another study, Maham et al. (2020) obtained green Ag/ZrO2 nanocomposites (50 nm) using Ageratum conyzoides plant extract. The catalyst could reduce and degrade a range of substrates such as 2,4-dinitrophenylhydrazine, 4-nitrophenol, nigrosin, and congo red, indicating the greatly catalytic performance of green Ag/ZrO2 nanocomposite.
Although green ZrO2 nanoparticles exhibit their very high catalytic performance, the separation and recovery of these non-magnetically small-size nanomaterials from the aqueous post-reaction is disadvantageous. The intercalation of magnetic components (e.g., Fe3O4) into ZrO2 nanomaterials should be therefore required. Inspired by this idea, Rostami-Vartooni et al. (2019) have successfully the Centaurea cyanus-biofabricated Ag/Fe3O4/ZrO2 nanocomposites with average particle size between 30 and 90 nm. The nanocomposite could be easily separated from the reaction solutions because its saturation magnetization value was obtained at 10 emu/g. The authors also reported some good results of catalytic reduction of 4-nitrophenol and degradation of methyl orange green Ag/Fe3O4/ZrO2 with a swift reaction time between 7.5 and 8 min. Therefore, this advantage may urge the recyclability experiments of magnetic ZrO2 nanocomposites.
ZrO2-based nanocomposites biosynthesized from the green extract sources can offer a high diversity of morphology and open new applications. For example, Pandiyan et al. (2018) employed the green synthesis of CeO2 doped ZrO2 nano sticks with small particle size (10–15 nm) and bandgap (3.37 eV) from Justicia adhatoda leaf extract. This nanocomposite exhibited high antibacterial activities against S. aureus, and E. coli and high antioxidant activity. They also reported the application of CeO2/ZrO2 nano sticks as potential biofilms against many pathogeneses caused by bacteria such as S. marcescens. Renuka et al. (2016) successfully created the hollow structure of Mg-doped ZrO2 microspheres using Aloe Vera leaf extract. They assumed that bio compounds such as polysaccharides from Aloe Vera leaf might chelate with Zr4+ and Mg2+ ions during the formation of Mg/ZrO2 microspheres. The authors assumed that bio compounds such as polysaccharides from Aloe Vera leaf might chelate with Zr4+ and Mg2+ ions during the reaction of complexation. The burning calcination of these complexes allowed to acquire the hollow structure of Mg/ZrO2 microspheres. They found the Mg/ZrO2 composites with the magnesium do** rate of 2 mol% gave the highest photocatalytic activity against Rhodamine B (93%) in the presence of ultraviolet irradiation. Controlled oxygen vacancies on active sites may be attributable to the enhanced catalytic performance of green Mg/ZrO2.
Perspective
Green synthesis of ZrO2 nanoparticles from microbial and botanical sources has gained great attention because they offer many advantages such as eco-friendly and low-cost manufacturing compared with chemical synthesis methods. Green ZrO2 nanoparticles possess many distinctive structure properties including small size, good dispersion, diverse surface chemistry, large surface area, and sufficient bandgap energy, making them extend excellent applications regarding biomedical and environmental fields. In many cases, the green ZrO2 nanoparticles experimentally exhibit better antibacterial, antifungal, catalytic and adsorptive performance than chemically synthesized ZrO2 nanomaterials. These superior prospects of green ZrO2 nanoparticles may pay the way for develo** advanced ZrO2-based nanosensors for smart agriculture, high-efficiency solar cells for energy harvesting, and electronic nanodevices for many high-technological manufacturing industries.
Although green ZrO2 nanoparticles hold many key advantages, there are still several limitations that need further investigation. Firstly, regarding the synthesis procedure for ZrO2 nanoparticles microbial sources, the control of biomass growth rate, metabolite generation, and biochemical properties of cultivation environment should be investigated vigorously. For the botanical sources, the determination of bioactive compound ingredients in the plant extract should be conducted to better elucidate the formation mechanism of ZrO2 nanoparticles. Moreover, the formation of green ZrO2 nanoparticles should be optimized to obtain their expectable properties of particle size, charge nature, surface energy, and surface chemistry. Secondly, regarding the treatment of pollutants such as antibiotics, and textile dyes, the treatment of reused ZrO2 nanoparticles has not been addressed. This action may generate secondary pollution; hence, it is necessary to solve the post-treatment problems. Moreover, most studies on the application of green ZrO2 nanoparticles only reported their findings under laboratory modes, resulting in the difference between simulated and practical results. This drawback can be therefore addressed if the tests are conducted under practical conditions. Thirdly, to the best of our knowledge, the potential of green ZrO2 nanoparticles is substantially unexploited in the many environmental fields, e.g., the treatment of emerging persistent organic pollutants, pharmaceuticals and personal care products, nonsteroidal anti-inflammatory drugs, and toxic gas detection. With the excellent properties of green ZrO2 nanoparticles, it is expected that they will contribute significantly to the development of new environmental remediation approaches. Ultimately, the combination of dopants with green ZrO2 nanoparticles can accelerate their inherent properties of the nanocomposites, expanding many promising applications. However, it is generally difficult to control the interactions of green extract with the dopants during the ZrO2 based nanocomposites. The optimization of the biomass proportion should be evaluated to avoid these interactions. Even though the formation mechanism and application of green ZrO2 based nanocomposites have been better understood in some published works, the number of researches is still limited. The knowledge of the overall interactions between ZrO2 nanomaterials and the environment is just burgeoning. The profound evaluations of the ecotoxicity of green ZrO2 nanoparticles should be continuously conducted to avoid possible future challenges.
Conclusion
The present review addressed some aspects of synthesis, properties, and potentials of green ZrO2 nanoparticles and their nanocomposites for biomedical, environmental remediation, and other applications. The bioactive compounds derived from microbial and botanical extracts are attributable to the bioreduction, biocap**, biochelating, and biostabilization during the transformation of zirconium sources into green ZrO2 nanoparticles. Through the structural characterization techniques, green ZrO2 nanoparticles are found with many morphologies such as nanospheres, nanorods, nano chains. They possess diverse surface chemistry, high stability, and wide bandgap energy possibly due to the effect of green extracts. Some applications regarding the antibacterial, antifungal, anticancer, photocatalytic, and adsorptive activities of green ZrO2 nanoparticles have been overviewed and discussed systematically. The advanced properties and applications of green ZrO2-based nanocomposites were also mentioned. To orientate the future researchers, some discussions of knowledge gaps and prospects of green ZrO2 nanoparticles have been clarified. The green ZrO2 nanoparticles and their nanocomposites are highly expected to obtain many good results in various applications.
Availability of data and material
The authors declare that all data and materials support their published claims and comply with field standards.
Code availability
The authors declare that software application or custom code supports their published claims and comply with field standards.
References
Abbasi E, Milani M, Fekri Aval S et al (2014) Silver nanoparticles: synthesis methods, bio-applications and properties. Crit Rev Microbiol 42:1–8. https://doi.org/10.3109/1040841X.2014.912200
Abinaya S, Kavitha HP, Prakash M, Muthukrishnaraj A (2021) Green synthesis of magnesium oxide nanoparticles and its applications: a review. Sustain Chem Pharm 19:100368. https://doi.org/10.1016/j.scp.2020.100368
Affonso LN, Marques JL, Lima VVC et al (2020) Removal of fluoride from fertilizer industry effluent using carbon nanotubes stabilized in chitosan sponge. J Hazard Mater 388:122042. https://doi.org/10.1016/j.jhazmat.2020.122042
Agarwal H, Venkat Kumar S, Rajeshkumar S (2017) A review on green synthesis of zinc oxide nanoparticles – An eco-friendly approach. Resour Technol 3:406–413. https://doi.org/10.1016/j.reffit.2017.03.002
Ahmed T, Ren H, Noman M et al (2021) Green synthesis and characterization of zirconium oxide nanoparticles by using a native Enterobacter sp. and its antifungal activity against bayberry twig blight disease pathogen Pestalotiopsis versicolor. NanoImpact 21:100281. https://doi.org/10.1016/j.impact.2020.100281
Akintelu SA, Folorunso AS (2020) A review on green synthesis of zinc oxide nanoparticles using plant extracts and its biomedical applications. Bionanoscience 10:848–863. https://doi.org/10.1007/s12668-020-00774-6
Alagarsamy A, Chandrasekaran S, Manikandan A (2022) Green synthesis and characterization studies of biogenic zirconium oxide (ZrO2) nanoparticles for adsorptive removal of methylene blue dye. J Mol Struct 1247:131275. https://doi.org/10.1016/j.molstruc.2021.131275
Alami AH, Aokal K, Zhang D et al (2019) Low-cost dye-sensitized solar cells with ball-milled tellurium-doped graphene as counter electrodes and a natural sensitizer dye. Int J Energy Res 43:5824–5833. https://doi.org/10.1002/er.4684
Al-Zaqri N, Muthuvel A, Jothibas M et al (2021) Biosynthesis of zirconium oxide nanoparticles using Wrightia tinctoria leaf extract: characterization, photocatalytic degradation and antibacterial activities. Inorg Chem Commun 127:108507. https://doi.org/10.1016/j.inoche.2021.108507
Annu A, Sivasankari C, Krupasankar U (2020) Synthesis and characerization of Zro2 nanoparticle by leaf extract bioreduction process for its biological studies. Mater Today Proc 33:5317–5323. https://doi.org/10.1016/j.matpr.2020.02.975
Bandeira M, Giovanela M, Roesch-Ely M et al (2020) Green synthesis of zinc oxide nanoparticles: a review of the synthesis methodology and mechanism of formation. Sustain Chem Pharm 15:100223. https://doi.org/10.1016/j.scp.2020.100223
Bansal V, Rautaray D, Ahmad A, Sastry M (2004) Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J Mater Chem 14:3303–3305. https://doi.org/10.1039/B407904C
Bansal V, Ramanathan R, Bhargava SK (2011) Fungus-mediated biological approaches towards ‘green’synthesis of oxide nanomaterials. Aust J Chem 64:279–293. https://doi.org/10.1071/CH10343
Beik J, Khateri M, Khosravi Z et al (2019) Gold nanoparticles in combinatorial cancer therapy strategies. Coord Chem Rev 387:299–324. https://doi.org/10.1016/j.ccr.2019.02.025
Böger B, Surek M, de O Vilhena R et al (2021) Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil. J Hazard Mater 402:123448. https://doi.org/10.1016/j.jhazmat.2020.123448
Bolade OP, Williams AB, Benson NU (2020) Green synthesis of iron-based nanomaterials for environmental remediation: a review. Environ Nanotechnol Monit Manag 13:100279. https://doi.org/10.1016/j.enmm.2019.100279
Cazado ME, Goldberg E, Togneri MA et al (2021) A new irradiation growth model for Zr-based components of nuclear reactors for the DIONISIO code. Nucl Eng Des 373:111009. https://doi.org/10.1016/j.nucengdes.2020.111009
Chau TP, Kandasamy S, Chinnathambi A et al (2021) Synthesis of zirconia nanoparticles using Laurus nobilis for use as an antimicrobial agent. Appl Nanosci. https://doi.org/10.1007/s13204-021-02041-w
Chen F, Wu Y-R, Wu J-M et al (2021) Preparation and characterization of ZrO2-Al2O3 bioceramics by stereolithography technology for dental restorations. Addit Manuf 44:102055. https://doi.org/10.1016/j.addma.2021.102055
Chu R, Li S, Zhu L et al (2021) A review on co-cultivation of microalgae with filamentous fungi: efficient harvesting, wastewater treatment and biofuel production. Renew Sustain Energy Rev 139:110689. https://doi.org/10.1016/j.rser.2020.110689
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
da Silva AFV, Fagundes AP, Macuvele DLP et al (2019) Green synthesis of zirconia nanoparticles based on Euclea natalensis plant extract: optimization of reaction conditions and evaluation of adsorptive properties. Colloids Surfaces A Physicochem Eng Asp 583:123915. https://doi.org/10.1016/j.colsurfa.2019.123915
Dai Y, Xu C, Sun X, Chen X (2017) Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev 46:3830–3852. https://doi.org/10.1039/C6CS00592F
Dang HH, Nguyen DTC, Nguyen TT et al (2021) Zeolitic-imidazolate framework-derived N-self-doped porous carbons with ultrahigh theoretical adsorption capacities for tetracycline and ciprofloxacin. J Environ Chem Eng 9:104938. https://doi.org/10.1016/j.jece.2020.104938
Davar F, Majedi A, Mirzaei A (2018) Polyvinyl alcohol thin film reinforced by green synthesized zirconia nanoparticles. Ceram Int 44:19377–19382. https://doi.org/10.1016/j.ceramint.2018.07.167
Debnath B, Majumdar M, Bhowmik M et al (2020) The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J Environ Manage 261:110235. https://doi.org/10.1016/j.jenvman.2020.110235
Dhandapani C, Narayanasamy R, Karthick SN et al (2016) Drastic photocatalytic degradation of methylene blue dye by neodymium doped zirconium oxide as photocatalyst under visible light irradiation. Optik (stuttg) 127:10288–10296. https://doi.org/10.1016/j.ijleo.2016.08.048
Fariq A, Khan T, Yasmin A (2017) Microbial synthesis of nanoparticles and their potential applications in biomedicine. J Appl Biomed 15:241–248. https://doi.org/10.1016/j.jab.2017.03.004
Ferreira JA, Varjani S, Taherzadeh MJ (2020) A critical review on the ubiquitous role of filamentous fungi in pollution mitigation. Curr Pollut Reports 6:295–309. https://doi.org/10.1007/s40726-020-00156-2
Filote C, Santos SCR, Popa VI et al (2021) Biorefinery of marine macroalgae into high-tech bioproducts: a review. Environ Chem Lett 19:969–1000. https://doi.org/10.1007/s10311-020-01124-4
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9:12944–12967. https://doi.org/10.1039/C8RA10483B
Ghomi GAR, Mohammadi-Khanaposhti M, Vahidi H et al (2019) Fungus-mediated extracellular biosynthesis and characterization of zirconium nanoparticles using standard penicillium species and their preliminary bactericidal potential: a novel biological approach to nanoparticle synthesis. Iran J Pharm Res 18:2101–2110
Gowri S, Gandhi RR, Sundrarajan M (2014) Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J Mater Sci Technol 30:782–790. https://doi.org/10.1016/j.jmst.2014.03.002
Gowri S, Gandhi RR, Senthil S, Sundrarajan M (2015) Effect of calcination temperature on nyctanthes plant mediated zirconia nanoparticles; optical and antibacterial activity for optimized zirconia. J Bionanoscience 9:181–189. https://doi.org/10.1166/jbns.2015.1297
Goyal P, Bhardwaj A, Mehta BK, Mehta D (2021) Research article green synthesis of zirconium oxide nanoparticles (ZrO2NPs) using Helianthus annuus seed and their antimicrobial effects. J Indian Chem Soc 98:100089. https://doi.org/10.1016/j.jics.2021.100089
Gurushantha K, Anantharaju KS, Sharma SC et al (2016) Bio-mediated Sm doped nano cubic zirconia: photoluminescent, Judd-Ofelt analysis, electrochemical impedance spectroscopy and photocatalytic performance. J Alloys Compd 685:761–773. https://doi.org/10.1016/j.jallcom.2016.06.105
Hamad SM, Mahmud SA, Sajadi SM, Omar ZA (2019) Biosynthesis of Cu/ZrO2 nanocomposite using 7-hydroxy-4´-methoxy-isoflavon extracted from Commelina diffusa and evaluation of its catalytic activity. Surfaces and Interfaces 15:125–134. https://doi.org/10.1016/j.surfin.2019.02.008
Hassan NS, Jalil AA (2022) A review on self-modification of zirconium dioxide nanocatalysts with enhanced visible-light-driven photodegradation of organic pollutants. J Hazard Mater 423:126996. https://doi.org/10.1016/j.jhazmat.2021.126996
Humbal C, Gautam S, Trivedi U (2018) A review on recent progress in observations, and health effects of bioaerosols. Environ Int 118:189–193. https://doi.org/10.1016/j.envint.2018.05.053
Indiarto R, Indriana LPA, Andoyo R et al (2021) Bottom–up nanoparticle synthesis: a review of techniques, polyphenol-based core materials, and their properties. Eur Food Res Technol. https://doi.org/10.1007/s00217-021-03867-y
Isacfranklin M, Dawoud T, Ameen F et al (2020) Synthesis of highly active biocompatible ZrO2 nanorods using a bioextract. Ceram Int 46:25915–25920. https://doi.org/10.1016/j.ceramint.2020.07.076
Jadoun S, Arif R, Jangid NK, Meena RK (2021) Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett 19:355–374. https://doi.org/10.1007/s10311-020-01074-x
Jameel MS, Aziz AA, Dheyab MA (2020) Comparative analysis of platinum nanoparticles synthesized using sonochemical-assisted and conventional green methods. Nano-Structures & Nano-Objects 23:100484. https://doi.org/10.1016/j.nanoso.2020.100484
Jiang H-L, Liu B, Lan Y-Q et al (2011) From metal-organic framework to nanoporous carbon: toward a very high surface area and hydrogen uptake. J Am Chem Soc 133:11854–11857. https://doi.org/10.1021/ja203184k
Jiang X, Nie X, Gong Y et al (2020) A combined experimental and DFT study of H2O effect on In2O3/ZrO2 catalyst for CO2 hydrogenation to methanol. J Catal 383:283–296. https://doi.org/10.1016/j.jcat.2020.01.014
Joshi NC, Chaudhary N, Rai N (2021) medicinal plant leaves extract based synthesis, characterisations and antimicrobial activities of ZrO2 nanoparticles (ZrO2 NPs). Bionanoscience 11:497–505. https://doi.org/10.1007/s12668-021-00829-2
Kairigo P, Ngumba E, Sundberg L-R et al (2020) Occurrence of antibiotics and risk of antibiotic resistance evolution in selected Kenyan wastewaters, surface waters and sediments. Sci Total Environ 720:137580. https://doi.org/10.1016/j.scitotenv.2020.137580
Kavitha NS, Venkatesh KS, Palani NS, Ilangovan R (2020) Synthesis and characterization of zirconium oxide nanoparticles using Fusarium solani extract. AIP Conf Proc 2265:30057. https://doi.org/10.1063/5.0017117
Khatoon N, Ahmad R, Sardar M (2015) Robust and fluorescent silver nanoparticles using Artemisia annua: biosynthesis, characterization and antibacterial activity. Biochem Eng J 102:91–97. https://doi.org/10.1016/j.bej.2015.02.019
Kovalakova P, Cizmas L, McDonald TJ et al (2020) Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere 251:126351. https://doi.org/10.1016/j.chemosphere.2020.126351
Kumaresan M, Vijai Anand K, Govindaraju K et al (2018) Seaweed Sargassum wightii mediated preparation of zirconia (ZrO2) nanoparticles and their antibacterial activity against gram positive and gram negative bacteria. Microb Pathog 124:311–315. https://doi.org/10.1016/j.micpath.2018.08.060
Li Z, Ma J, Ruan J, Zhuang X (2019) Using positively charged magnetic nanoparticles to capture bacteria at ultralow concentration. Nanoscale Res Lett 14:195. https://doi.org/10.1186/s11671-019-3005-z
Maham M, Nasrollahzadeh M, Mohammad Sajadi S (2020) Facile synthesis of Ag/ZrO2 nanocomposite as a recyclable catalyst for the treatment of environmental pollutants. Compos Part B Eng 185:107783. https://doi.org/10.1016/j.compositesb.2020.107783
Majedi A, Abbasi A, Davar F (2016) Green synthesis of zirconia nanoparticles using the modified Pechini method and characterization of its optical and electrical properties. J Sol-Gel Sci Technol 77:542–552. https://doi.org/10.1007/s10971-015-3881-3
Makuła P, Pacia M, Macyk W (2018) How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J Phys Chem Lett 9:6814–6817. https://doi.org/10.1021/acs.jpclett.8b02892
Mooney R, Hammad M, Batalla-Covello J et al (2018) Concise review: neural stem cell-mediated targeted cancer therapies. Stem Cells Transl Med 7:740–747. https://doi.org/10.1002/sctm.18-0003
Muhammad S, Long X, Salman M (2020) COVID-19 pandemic and environmental pollution: A blessing in disguise? Sci Total Environ 728:138820. https://doi.org/10.1016/j.scitotenv.2020.138820
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13. https://doi.org/10.1016/j.cis.2010.02.001
Nguyen DTC, Dang HH, Vo D-VN et al (2021) Biogenic synthesis of MgO nanoparticles from different extracts (flower, bark, leaf) of Tecoma stans (L.) and their utilization in selected organic dyes treatment. J Hazard Mater 404:124146. https://doi.org/10.1016/j.jhazmat.2020.124146
Nguyen DTC, Le HTN, Nguyen TT et al (2021b) Engineering conversion of Asteraceae plants into biochars for exploring potential applications: a review. Sci Total Environ 797:149195. https://doi.org/10.1016/j.scitotenv.2021.149195
Nguyen DTC, Le HTN, Nguyen TT et al (2021) Multifunctional ZnO nanoparticles bio-fabricated from Canna indica L. flowers for seed germination, adsorption, and photocatalytic degradation of organic dyes. J Hazard Mater 420:126586. https://doi.org/10.1016/j.jhazmat.2021.126586
Nguyen DTC, Nguyen TT, Le HTN et al (2021d) The sunflower plant family for bioenergy, environmental remediation, nanotechnology, medicine, food and agriculture. Rev Environ Chem Lett 19:3701–3726. https://doi.org/10.1007/s10311-021-01266-z
Nguyen DTC, Vo D-VN, Nguyen CNQ et al (2021e) Box-Behnken design, kinetic, and isotherm models for oxytetracycline adsorption onto Co-based ZIF-67. Appl Nanosci 11:2347–2359. https://doi.org/10.1007/s13204-021-01954-w
Nguyen LTT, Vo D-VN, Nguyen LTH et al (2021f) Synthesis, characterization, and application of ZnFe2O4@ZnO nanoparticles for photocatalytic degradation of Rhodamine B under visible-light illumination. Environ Technol Innov. https://doi.org/10.1016/j.eti.2021.102130
Oberoi AS, Jia Y, Zhang H et al (2019) Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ Sci Technol 53:7234–7264. https://doi.org/10.1021/acs.est.9b01131
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew Sustain Energy Rev 81:536–551. https://doi.org/10.1016/j.rser.2017.08.020
Pandiyan N, Murugesan B, Sonamuthu J et al (2018) Facile biological synthetic strategy to morphologically aligned CeO2/ZrO2 core nanoparticles using Justicia adhatoda extract and ionic liquid: Enhancement of its bio-medical properties. J Photochem Photobiol B Biol 178:481–488. https://doi.org/10.1016/j.jphotobiol.2017.11.036
Patil MP, Kim G-D (2018) Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf B Biointerfaces 172:487–495. https://doi.org/10.1016/j.colsurfb.2018.09.007
Ponce NMA, Stortz CA (2020) A comprehensive and comparative analysis of the fucoidan compositional data across the phaeophyceae. Front Plant Sci 11:1844. https://doi.org/10.3389/fpls.2020.556312
Prasad KS, Amin Y, Selvaraj K (2014) Defluoridation using biomimetically synthesized nano zirconium chitosan composite: kinetic and equilibrium studies. J Hazard Mater 276:232–240. https://doi.org/10.1016/j.jhazmat.2014.05.038
Ram S, Mitra M, Shah F et al (2020) Bacteria as an alternate biofactory for carotenoid production: a review of its applications, opportunities and challenges. J Funct Foods 67:103867. https://doi.org/10.1016/j.jff.2020.103867
Rambabu K, Bharath G, Arangadi AF et al (2020) ZrO2 incorporated polysulfone anion exchange membranes for fuel cell applications. Int J Hydrogen Energy 45:29668–29680. https://doi.org/10.1016/j.ijhydene.2020.08.175
Rana A, Yadav K, Jagadevan S (2020) A comprehensive review on green synthesis of nature-inspired metal nanoparticles: mechanism, application and toxicity. J Clean Prod 272:122880. https://doi.org/10.1016/j.jclepro.2020.122880
Ranji-Burachaloo H, Gurr PA, Dunstan DE, Qiao GG (2018) Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano 12:11819–11837. https://doi.org/10.1021/acsnano.8b07635
Rasheed P, Haq S, Waseem M et al (2020) Green synthesis of vanadium oxide-zirconium oxide nanocomposite for the degradation of methyl orange and picloram. Mater Res Express 7:25011. https://doi.org/10.1088/2053-1591/ab6fa2
Reddy GB, Madhusudhan A, Ramakrishna D et al (2015) Green chemistry approach for the synthesis of gold nanoparticles with gum kondagogu: characterization, catalytic and antibacterial activity. J Nanostructure Chem 5:185–193. https://doi.org/10.1007/s40097-015-0149-y
Renuka L, Anantharaju KS, Sharma SC et al (2016) Hollow microspheres Mg-doped ZrO2 nanoparticles: green assisted synthesis and applications in photocatalysis and photoluminescence. J Alloys Compd 672:609–622. https://doi.org/10.1016/j.jallcom.2016.02.124
Riaz M, Kamran M, Fang Y et al (2021) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 402:123919. https://doi.org/10.1016/j.jhazmat.2020.123919
Rostami-Vartooni A, Moradi-Saadatmand A, Bagherzadeh M, Mahdavi M (2019) Green synthesis of Ag/Fe3O4/ZrO2 nanocomposite using aqueous Centaurea cyanus flower extract and its catalytic application for reduction of organic pollutants. Iran J Catal 9:27–35
Roy P, Kumar Sinha N, Tiwari S, Khare A (2020) A review on perovskite solar cells: evolution of architecture, fabrication techniques, commercialization issues and status. Sol Energy 198:665–688. https://doi.org/10.1016/j.solener.2020.01.080
Rozana M, Soaid NI, Kian TW et al (2017) Photocatalytic performance of freestanding tetragonal zirconia nanotubes formed in H2O2/NH4F/ethylene glycol electrolyte by anodisation of zirconium. Nanotechnology 28:155604. https://doi.org/10.1088/1361-6528/aa5fac
Saraswathi VS, Santhakumar K (2017) Photocatalytic activity against azo dye and cytotoxicity on MCF-7 cell lines of zirconium oxide nanoparticle mediated using leaves of Lagerstroemia speciosa. J Photochem Photobiol B Biol 169:47–55. https://doi.org/10.1016/j.jphotobiol.2017.02.023
Saravanan A, Kumar PS, Karishma S et al (2021) A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 264:128580. https://doi.org/10.1016/j.chemosphere.2020.128580
Sathishkumar M, Sneha K, Yun Y-S (2013) Green fabrication of zirconia nano-chains using novel Curcuma longa tuber extract. Mater Lett 98:242–245. https://doi.org/10.1016/j.matlet.2013.02.036
Shafey AME (2020) Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process Synth 9:304–339. https://doi.org/10.1515/gps-2020-0031
Shinde HM, Bhosale TT, Gavade NL et al (2018) Biosynthesis of ZrO2 nanoparticles from Ficus benghalensis leaf extract for photocatalytic activity. J Mater Sci Mater Electron 29:14055–14064. https://doi.org/10.1007/s10854-018-9537-7
Shrimal P, Jadeja G, Patel S (2020) A review on novel methodologies for drug nanoparticle preparation: microfluidic approach. Chem Eng Res Des 153:728–756. https://doi.org/10.1016/j.cherd.2019.11.031
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnology 15:65. https://doi.org/10.1186/s12951-017-0308-z
Srivastava M, Srivastava N, Mishra PK, Malhotra BD (2021) Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci Total Environ 754:142363. https://doi.org/10.1016/j.scitotenv.2020.142363
Sudrajat H, Babel S, Sakai H, Takizawa S (2016) Rapid enhanced photocatalytic degradation of dyes using novel N-doped ZrO2. J Environ Manage 165:224–234. https://doi.org/10.1016/j.jenvman.2015.09.036
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Suresh D, Nethravathi PC, Rajanaika H et al (2015) Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater Sci Semicond Process 31:446–454. https://doi.org/10.1016/j.mssp.2014.12.023
Suriyaraj SP, Ramadoss G, Chandraraj K, Selvakumar R (2019) One pot facile green synthesis of crystalline bio-ZrO2 nanoparticles using Acinetobacter sp. KCSI1 under room temperature. Mater Sci Eng C 105:110021. https://doi.org/10.1016/j.msec.2019.110021
Tahrani L, Van Loco J, Ben Mansour H, Reyns T (2015) Occurrence of antibiotics in pharmaceutical industrial wastewater, wastewater treatment plant and sea waters in Tunisia. J Water Health 14:208–213. https://doi.org/10.2166/WH.2015.224
Tang Z, Zhang X, Shu Y et al (2021) Insights from nanotechnology in COVID-19 treatment. Nano Today 36:101019. https://doi.org/10.1016/j.nantod.2020.101019
Tran TV, Nguyen DTC, Le HTN et al (2019a) Facile synthesis of manganese oxide-embedded mesoporous carbons and their adsorbability towards methylene blue. Chemosphere 227:455–461. https://doi.org/10.1016/j.chemosphere.2019.04.079
Tran TV, Nguyen DTC, Le HTN et al (2019b) Response surface methodology-optimized removal of chloramphenicol pharmaceutical from wastewater using Cu3(BTC)2-derived porous carbon as an efficient adsorbent. Comptes Rendus Chim 22:794–803. https://doi.org/10.1016/j.crci.2019.09.004
Tran TV, Nguyen DTC, Nguyen TT et al (2020a) High performance of Mn2(BDC)2(DMF)2-derived MnO@C nanocomposite as superior remediator for a series of emergent antibiotics. J Mol Liq 308:113038. https://doi.org/10.1016/j.molliq.2020.113038
Tran TV, Nguyen DTC, Nguyen TT et al (2020b) Metal-organic framework HKUST-1-based Cu/Cu2O/CuO@C porous composite: rapid synthesis and uptake application in antibiotics remediation. J Water Process Eng 36:101319. https://doi.org/10.1016/j.jwpe.2020.101319
Tran TV, Nguyen H, Le PHA et al (2020c) Microwave-assisted solvothermal fabrication of hybrid zeolitic–imidazolate framework (ZIF-8) for optimizing dyes adsorption efficiency using response surface methodology. J Environ Chem Eng 8:104189. https://doi.org/10.1016/j.jece.2020.104189
Tran TV, Nguyen VH, Nong LX et al (2020d) Hexagonal Fe-based MIL-88B nanocrystals with NH2 functional groups accelerating oxytetracycline capture via hydrogen bonding. Surf Interfaces 20:100605. https://doi.org/10.1016/j.surfin.2020.100605
Tran TV, Phan T-QT, Nguyen DTC et al (2020e) Recyclable Fe3O4@ C nanocomposite as potential adsorbent for a wide range of organic dyes and simulated hospital effluents. Environ Technol Innov 20:101122. https://doi.org/10.1016/j.eti.2020.101122
Tran TV, Nong LX, Nguyen H-TT et al (2021) Response surface methodology modeling for methylene blue removal by chemically modified porous carbon: adsorption mechanism and role of surface functional groups. Sep Sci Technol 56:2232–2242. https://doi.org/10.1080/01496395.2020.1820523
Ufnalska S, Lichtfouse E (2021) Unanswered issues related to the COVID-19 pandemic. Environ Chem Lett 19:3523–3524. https://doi.org/10.1007/s10311-021-01249-0
Vennila R, Kamaraj P, Arthanareeswari M et al (2018) Biosynthesis of ZrO nanoparticles and its natural dye sensitized solar cell studies. Mater Today Proc 5:8691–8698. https://doi.org/10.1016/j.matpr.2017.12.295
Vijayaraghavan K, Ashokkumar T (2017) Plant-mediated biosynthesis of metallic nanoparticles: a review of literature, factors affecting synthesis, characterization techniques and applications. J Environ Chem Eng 5:4866–4883. https://doi.org/10.1016/j.jece.2017.09.026
Vivekanandhan S, Venkateswarlu M, Rawls HR et al (2015) Synthesis and characterization of AgNP: ZrO2 functional nanomaterials by leaf extract assisted bioreduction process. Ceram Int 41:3305–3311. https://doi.org/10.1016/j.ceramint.2014.10.111
Wang M, Chen Z, Liu J et al (2021) Advanced high-temperature (RT-1100°C) resistant adhesion technique for joining dissimilar ZrO2 ceramic and TC4 superalloys based on an inorganic/organic hybrid adhesive. Ceram Int. https://doi.org/10.1016/j.ceramint.2021.10.083
Weiss C, Carriere M, Fusco L et al (2020) Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 14:6383–6406. https://doi.org/10.1021/acsnano.0c03697
**ng X, Ma W, Zhao X et al (2018) Interaction between surface charge-modified gold nanoparticles and phospholipid membranes. Langmuir 34:12583–12589. https://doi.org/10.1021/acs.langmuir.8b01700
Xu C, Huang S, Huang Y et al (2020) New insights into the harmful algae inhibition by Spartina alterniflora: cellular physiology and metabolism of extracellular secretion. Sci Total Environ 714:136737. https://doi.org/10.1016/j.scitotenv.2020.136737
Yadav LSR, Ramakrishnappa T, Rayan Pereira J et al (2021) Rubber latex fuel extracted green biogenic nickel doped ZrO2 nanoparticles and its resistivity. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.05.171
Yadi M, Mostafavi E, Saleh B et al (2018) Current developments in green synthesis of metallic nanoparticles using plant extracts: a review. Artif Cells, Nanomed Biotechnol 46:S336–S343. https://doi.org/10.1080/21691401.2018.1492931
Yang NJ, Chiu IM (2017) Bacterial signaling to the nervous system through toxins and metabolites. J Mol Biol 429:587–605. https://doi.org/10.1016/j.jmb.2016.12.023
Zhang Y, Chen H-X, Duan L et al (2018) A comparison study of the structural and mechanical properties of cubic, tetragonal, monoclinic, and three orthorhombic phases of ZrO2. J Alloys Compd 749:283–292. https://doi.org/10.1016/j.jallcom.2018.03.253
Zhou Y, Lu J, Zhou Y, Liu Y (2019) Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut 252:352–365. https://doi.org/10.1016/j.envpol.2019.05.072
Acknowledgments
The authors would love to appreciate the effort of researchers all over the world in the fight against the COVID-19 pandemic.
Funding
There was no external funding for this study.
Author information
Authors and Affiliations
Contributions
TVT contributed to Conceptualisation; Data curation; Investigation; Methodology; Writing—original draft; Writing—review & editing. DTCN contributed to Conceptualisation; Data curation; Investigation; Methodology; Writing—original draft; Writing—review & editing. PSK contributed to Writing—review & editing; Data curation; Supervision. ATMD contributed to Writing—review & editing; Data curation; Supervision. AAJ contributed to Writing—review & editing; Data curation; Supervision. D-VNV contributed to Writing—review & editing; Data curation; Supervision; Project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Consent for publication
The manuscript has not been published anywhere nor submitted to another journal. The manuscript is not currently being considered for publication in any another journal. All authors have been personally and actively involved in substantive work leading to the manuscript, and will hold themselves jointly and individually responsible for its content.
Human and animal rights
Research does not involve any Human Participants and/or Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tran, T.V., Nguyen, D.T.C., Kumar, P.S. et al. Green synthesis of ZrO2 nanoparticles and nanocomposites for biomedical and environmental applications: a review. Environ Chem Lett 20, 1309–1331 (2022). https://doi.org/10.1007/s10311-021-01367-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01367-9