Abstract
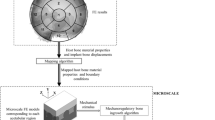
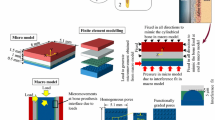
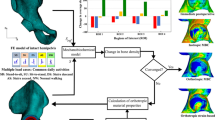
Fixation of uncemented implant is influenced by peri-prosthetic bone ingrowth, which is dependent on the mechanical environment of the implant–bone structure. The objective of the study is to gain an insight into the tissue differentiation around an acetabular component. A map** framework has been developed to simulate appropriate mechanical environment in the three-dimensional microscale model, implement the mechanoregulatory tissue differentiation algorithm and subsequently assess spatial distribution of bone ingrowth around an acetabular component, quantitatively. The FE model of implanted pelvis subjected to eight static load cases during a normal walking cycle was first solved. Thereafter, a map** algorithm has been employed to include the variations in implant–bone relative displacement and host bone material properties from the macroscale FE model of implanted pelvis to the microscale FE model of the beaded implant–bone interface. The evolutionary tissue differentiation was observed in each of the 13 microscale models corresponding to 13 acetabular regions. The total implant–bone relative displacements, averaged over each region of the acetabulum, were found to vary between 10 and 60 \(\upmu \hbox {m}\). Both the linear elastic and biphasic poroelastic models predicted similar mechanoregulatory peri-prosthetic tissue differentiation. Considerable variations in bone ingrowth (13–88 %), interdigitation depth (0.2–0.82 mm) and average tissue Young’s modulus (970–3430 MPa) were predicted around the acetabular cup. A progressive increase in the average Young’s modulus, interdigitation depth and decrease in average radial strains of newly formed tissue layer were also observed. This scheme can be extended to investigate tissue differentiation for different surface texture designs on the implants.






Similar content being viewed by others
References
Anderson AE, Peters CL, Tuttle BD, Weiss JA (2005) Subject-specific finite element model of the pelvis: development, validation, sensitive studies. J Biomech Eng 127(3):364–373
Andreykiv A, van Keulen F, Prendergast PJ (2007) Simulation of fracture healing incorporating mechanoregulation of tissue differentiation and dispersal/proliferation of cells. Biomech Model Mechanobiol 7(6):443–461
Armstrong CG, Mow VC (1982) Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am 64–A–1:88–94
Atkinson J (ed) (2007) Pore pressure, effective stress and drainage. In: The mechanics of soils and foundations, 2nd edn. Taylor & Francis, New York, pp 75–77
Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN (2001) Hip contact forces and gait patterns from routine activities. J Biomech 34(7):859–871
Besong AA, Lee R, Farrar R, ** ZM (2001) Contact mechanics of a novel metal-on-metal total hip replacement. Proc Inst Mech Eng H 215(6):543–548
Biot MA (1941) General theory of three-dimensional consolidation. J Appl Phys 12:155–164
Bragdon CR, Jasty M, Greene M, Rubash HE, Harris WH (2004) Biologic fixation of total hip implants: insights gained from a series of canine studies. J Bone Joint Surg Am 86–A(Suppl 2):105–117
Caouette C, Bureau MN, Lavigne M, Vendittoli PA, Nuño N (2013) A new interface element with progressive damage and osseointegration for modeling of interfaces in hip resurfacing. Proc Inst Mech Eng H 227(3):209–220
Carter DR, Blenman PR, Beaupre GS (1988) Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J Orthop Res 6(5):736–748
Checa S, Prendergast PJ (2009) A mechanobiological model for tissue differentiation that includes angiogenesis: a lattice-based modeling approach. Ann Biomed Eng 37(1):129–145
Chou HY, Müftü S (2013) Simulation of peri-implant bone healing due to immediate loading in dental implant treatments. J Biomech 46(14):871–878
Claes L, Augat P, Suger G, Wilke HJ (1997) Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res 15(4):577–584
Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P (1998) Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res 355(Suppl):S132–S147
Claes LE, Heigele CA (1999) Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech 32(3):255–266
Clarke SG, Phillips ATM, Bull AMJ (2013) Evaluating a suitable level of model complexity for finite element analysis of the intact acetabulum. Comput Methods Biomech Biomed Eng 16(7):717–724
Dalstra M, Huiskes R (1995) Load transfer across the pelvis bone. J Biomech 28(6):715–724
Davies JE (1996) In vitro modeling of the bone/implant interface. Anat Rec 245(2):426–445
Davies JE (2003) Understanding peri-implant endosseous healing. J Dent Educ 67(8):932–949
Dickinson A, Taylor A, Browne M (2012) Implant-bone interface healing and adaptation in resurfacing hip replacement. Comput Methods Biomech Biomed Eng 15(9):935–947
Dostal WF, Andrews JG (1981) A three-dimensional biomechanical model of hip musculature. J Biomech 14(11):803–812
Engh CA, Bobyn JD, Glassman AH (1987) Porous-coated hip replacement: the factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br 69-B(1):45–55
Engh CA, Zettl-Schaffer KF, Kukita Y, Sweet D, Jasty M, Bragdon C (1993) Histological and radiographic assessment of well functioning porous-coated acetabular components. A human postmortem retrieval study. J Bone Joint Surg Am 75-A(6):814–824
Fini M, Giavaresi G, Rimondini L, Giardino R (2002) Titanium alloy osseointegration in cancellous and cortical bone of ovariectomized animals: histomorphometric and bone hardness measurements. Int J Oral Maxillofac Implants 17(1):28–37
Furlong RJ, Osborn JF (1991) Fixation of hip prostheses by hydroxyapatite ceramic coatings. J Bone Joint Surg Br 73-B(5):741–745
Ghosh R, Gupta S (2014) Bone remodelling around cementless composite acetabular components: the effects of implant geometry and implant-bone interfacial conditions. J Mech Behav Biomed Mater 32:257–269
Ghosh R, Gupta S, Dickinson A, Browne M (2012) Experimental validation of finite element models of intact and implanted composite hemi-pelvises using digital image correlation. J Biomech Eng 134(8):1–9
Ghosh R, Gupta S, Dickinson A, Browne M (2013a) Experimental validation of numerically predicted strain and micromotion in intact and implanted composite hemi-pelvises. Proc Inst Mech Eng H 227(2):162–174
Ghosh R, Mukherjee K, Gupta S (2013) Bone remodelling around uncemented metallic and ceramic acetabular components. Proc Inst Mech Eng H 227(5):490–502. doi:10.1177/0954411913478703
Ghosh R, Pal B, Ghosh D, Gupta S (2015) Finite element analysis of a hemi-pelvis: the effect of inclusion of cartilage layer on acetabular stresses and strain. Comput Methods Biomech Biomed Eng 18(7):697–710. doi:10.1080/10255842.2013.843674
Haddad RJ, Cook SD, Thomas KA (1987) Biological fixation of porous-coated implants. J Bone Joint Surg Am 69-A(9):1459–1466
Hanzlik JA, Day JS (2013) Bone ingrowth in well-fixed retrieved porous tantalum implants. J Arthroplasty 28:922–927
Hollister SJ, Guldberg RE, Kuelske CL, Caldwell NJ, Richards M, Goldstein SA (1996) Relative effects of wound healing and mechanical stimulus on early bone response to porous-coated implants. J Orthop Res 14(4):654–662
Hori RY, Lewis JL (1982) Mechanical properties of the fibrous tissue found at the bone-cement interface following total joint replacement. J Biomed Mater Res 16(6):911–927
Hothi HS (2012) The impact and deformation of press-fit metal acetabular components. Dissertation, Queen Mary University of London, UK
Huiskes R, van Driel WD, Prendergast PJ, Soballe K (1997) A biomechanical regulatory model for periprosthetic fibrous tissue differentiation. J Mater Sci Mater Med 8(12):785–788
Isaksson H, van Donkelaar CC, Huiskes R, Ito K (2008) A mechano-regulatory bone-healing model incorporating cell-phenotype specific activity. J Theor Biol 252(2):230–246
Isaksson H, Wilson W, van Donkelaar CC, Huiskes R, Ito K (2006) Comparison of biophysical stimuli for mechanoregulation of tissue differentiation during fracture healing. J Biomech 39(8):1507–1516
Janssen D, Zwartele RE, Doets HC, Verdonschot N (2010) Computational assessment of press-fit acetabular implant fixation: the effect of implant design, interference fit, bone quality, and frictional properties. Proc Inst Mech Eng H 224(1):65–75
Jasty M, Bragdon CR, Burke D, O’Connor D, Lowenstein J, Harris WH (1997) In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg Am 79–A(5):707–714
Jasty M, Bragdon CR, Maloney WJ, Haire T, Harris WH (1991) Ingrowth of bone in failed fixation of porous-coated femoral components. J Bone Joint Surg Am 73-A(9):1331–1337
Jurvelin JS, Buschmann MD, Hunziker EB (1997) Optical and mechanical determination of Poisson’s ratio of adult bovine humeral articular cartilage. J Biomech 30(3):235–241
Kuzyk PR, Schemitsch EH (2011) The basic science of peri-implant bone healing. Indian J Orthop 45(2):108–115
Lacroix D, Prendergast PJ (2002a) A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech 35(9):1163–1171
Lacroix D, Prendergast PJ (2002b) Three-dimensional simulation of fracture repair in the human tibia. Comput Methods Biomech Biomed Eng 5(5):369–376
Lacroix D, Prendergast PJ, Li G, Marsh D (2002) Biomechanical model to simulate tissue differentiation and bone regeneration: application to fracture healing. Med Biol Eng Comput 40(1):14–21
Levenston ME, Beaupre GS, Schurman DJ, Carter DR (1993) Computer simulations of stress-related bone remodelling around noncemented acetabular components. J Arthroplasty 8(6):595–605
Liu F, ** Z, Roberts P, Grigoris P (2006) Importance of head diameter, clearance and cup wall thickness in elastohydrodynamic lubrication analysis of metal-on-metal hip resurfacing prostheses. Proc Inst Mech Eng H 220(6):695–704
Liu X, Niebur GL (2008) Bone ingrowth into a porous coated implant predicted by a mechano-regulatory tissue differentiation algorithm. Biomech Model Mechanobiol 7(4):335–344
Manley MT, Ong KL, Kurtz SM (2006) The potential for bone loss in acetabular structures following THA. Clin Orthop Relat Res 453:246–253
Morrison ML (2006) Birmingham hip resurfacing system. Adv Mater Process 164(10):52–53
Mukherjee K, Gupta S (2014) Simulation of tissue differentiation around acetabular cups: the effects of implant-bone relative displacement and polar gap. Adv Biomech Appl 1(2):95–109
Ochoa JA, Hillberry BM (1992) Permeability of bovine cancellous bone. In: Proceedings of the 38th annual meeting of the orthopaedic research society, Washington, DC
Ong KL, Lehman J, Notz WI, Santner TJ, Bartel DL (2006) Acetabular cup geometry and bone-implant interface have more influence on initial periprosthetic joint space than joint loading and surgical cup insertion. J Biomech Eng 128(2):169–175
Pienkowski D, Pollack SR (1983) The origin of stress generated potentials in fluid saturated bone. J Orthop Res 1:30–41
Prendergast PJ, Huiskes R, Søballe K (1997) Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech 30(6):539–548
Puleo DA, Nanci A (1999) Understanding and controlling the bone-implant interface. Biomaterials 20(23–24):2311–2321
Puthumanapully PK (2010) Simulation of tissue differentiation in uncemented hip implants based on a mechanoregulatory hypothesis. Ph.D. Dissertation, University of Southampton, UK
Puthumanapully PK, Browne M (2011) Tissue differentiation around a short stemmed metaphyseal loading implant employing a modified mechanoregulatory algorithm: a finite element study. J Orthop Res 29(5):787–794
Shih LY, Shih HN, Chen TH (2003) The effects of sex and estrogen therapy on bone ingrowth into porous coated implant. J Orthop Res 21(6):1033–1040
Smit TH, Huyghe JM, Cowin SC (2002) Estimation of the poroelastic parameters of cortical bone. J Biomech 35(6):829–835
Spears IR, Pfleiderer M, Schneider E, Hailee E, Morlock MM (2001) The effect of interfacial parameters on cup-bone relative micromotions: a finite element investigation. J Biomech 34(1):113–120
Sumner DR, Jasty M, Jacobs JJ, Urban RM, Bragdon CR, Harris WH, Galante JO (1993) Histology of porous-coated acetabular components. 25 cementless cups retrieved after arthroplasty. Acta Orthop Scand 64(6):619–626
Taddei F, Pancanti A, Viceconti M (2004) An improved method for the automatic map** of computed tomography numbers onto finite element models. Med Eng Phys 26(1):61–69
Tarala M, Waanders D, Biemond JE, Hannink G, Janssen D, Buma P, Verdonschot N (2011) The effect of bone ingrowth depth on the tensile and shear strength of the implant-bone e-beam produced interface. J Mater Sci Mater Med 22(10):2339–2346
Thompson MS, Northmore-Ball MD, Tanner KE (2002) Effect of acetabular resurfacing component material and fixation on the strain distribution in the pelvis. Proc Inst Mech Eng H 216(4):237–245
Udofia IJ, Yew A, ** JM (2004) Contact mechanics analysis of metal-on-metal hip resurfacing prostheses. Proc Inst Mech Eng H 218(5):293–305
US Food and Drug Administration (FDA) (2006) Birmingham hip resurfacing (BHR) system: summary of safety and effectiveness data. http://www.accessdata.fda.gov/cdrh_docs/pdf4/P040033b.pdf. Accessed 21 Aug 2014
Widmer KH, Zurfluh B, Morscher EW (2002) Load transfer and fixation mode of press-fit acetabular sockets. J Arthroplasty 17:926–935
Yew A, ** ZM, Donn A, Morlock MM, Isaac G (2006) Deformation of press-fitted metallic resurfacing cups part 2: finite element simulation. Proc Inst Mech Eng H 220(2):311–319
Zhang QH, Wang JY, Lupton C, Adegbile PH, Guo ZX, Liu Q, Tong J (2010) A subject-specific pelvic bone model and its application to cemented acetabular replacements. J Biomech 43(14):2722–2727
Acknowledgments
The authors would like to specially thank Dr. D. K. Nanda and Mr. A. Chattopadhyay of Computer and Informatics Centre, IIT Kharagpur for extending computational infrastructure required for the study. The authors would also like to thank University of Southampton, UK, for providing CT scan of the patient under the UKIERI collaborative research project funded by British Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, K., Gupta, S. Bone ingrowth around porous-coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol 15, 389–403 (2016). https://doi.org/10.1007/s10237-015-0696-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0696-7