Abstract
Background
In pediatric patients, due to variations in baseline serum creatinine (Cr) reference values, renal dysfunctions sometimes go unnoticed. In addition, renally excreted drugs need dose adjustment while nephrotoxic drugs should be avoided altogether in patients with impaired renal function. However, most physicians are apparently unaware of these facts and may administer these drugs to vulnerable patients.
Methods
We administered a questionnaire to all physicians and pharmacists specializing in pediatric medical care at six Tokyo metropolitan government-run hospitals in Japan.
Results
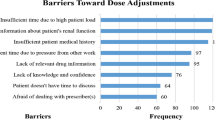
276 (59%) of 470 physicians and pharmacists participated. The rate of correct answers given by physicians who were asked to state the serum Cr reference range for 4-year-olds and 8-year-olds was 83 and 74%, respectively. On the other hand, the rate of correct answers given by pharmacists to the same question was only 27 and 24%, respectively. Only about 50% of physicians were aware that histamine H2-receptor antagonists and oseltamivir are renally excreted or that acyclovir and angiotensin II receptor blocker are nephrotoxic. However, most of the pharmacists recognized that histamine H2-receptor antagonists and oseltamivir are renally excreted drugs.
Conclusions
For the majority of the investigated drugs, the awareness that we need to reduce dosages for patients with renal dysfunction was insufficient. To ensure safe drug administration, communication between physicians and pharmacists is paramount. There is an urgent need for the creation of a safe drug administration protocol for pediatric patients with renal dysfunction.


Similar content being viewed by others
References
Hug BL, Witkowski DJ, Sox CM, Keohane CA, Segar DL, Yoon C, Matheny ME, Bates DW. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009;76:1192–8.
Silva DC, Araujo OR, Arduini RG, Alonso CF, Shibata AR, Troster EJ. Adverse drug events in a paediatric intensive care unit: a prospective cohort. BMJ Open. 2013;19:e001868.
Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–20.
Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4:1275–83.
Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53:2887–91.
Zappitellu M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transpl. 2011;26:144–50.
Kelkar M, Cleves MA, Foster HR, Hogan WR, James LP, Martin BC. Acute and chronic acetaminophen use and renal disease: a case-control study using pharmacy and medical claims. J Manag Care Pharm. 2012;18:234–46.
Marenzi G, Cabiati A, Milazzo V, Rubino M. Contrast-induced nephropathy. Intern Emerg Med. 2012;7:S181–3.
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Yata N, Nagai T, Ikezumi Y, Fujita N, Ito S, Iijima K, Kitagawa T. Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: a multicenter study. Clin Exp Nephrol. 2011;15:694–9.
Ishikura K, Uemura O, Ito S, Wada N, Hattori M, Ohashi Y, Hamasaki Y, Tanaka R, Nakanishi K, Kaneko T, Honda M, Pediatric CKD. Study Group, Japan Committee of Measures for Pediatric CKD of the Japanese Society of Pediatric Nephrology. Pre-dialysis chronic kidney disease in children: results of a nationwide survey in Japan. Nephrol Dial Transpl. 2013;28:2345–55.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–33.
Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S.
Humes HD. Aminoglycoside nephrotoxicity. Kidney Int. 1988;33:900–11.
Perazella MA. Crystal-induced acute renal failure. Am J Med. 1999;106:459–65.
Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Eng J Med. 1989;320:143–9.
Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J Pharmacol Exp Ther. 1980;213:551–6.
Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Platt R. Correlates of acute renal failure in patients receiving parenteral amphotericin B. Kidney Int. 2001;60:1452–9.
Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–93.
Lee HY, Kim CH. Acute oliguric renal failure associated with angiotensin II receptor antagonists. Am J Med. 2001;111:162–3.
Saleem A, Masood I. Pattern and predictors of medication dosing errors in chronic kidney disease patients in Pakistan: a single center retrospective analysis. PLoS One. 2016;11:e0158677.
Redmond AM, Pentapaty N, Weibel J, Nolan SF, Hudson JQ, Self T. Use of famotidine in adult patients with end-stage renal disease: assessment of dosing and mental status changes. Am J Med Sci. 2005;330:8–10.
Hartmann B, Czock D, Keller F. Drug therapy in patients with chronic renal failure. Dtsch Arztebl Int. 2010;107:647–55.
Daschner M. Drug dosage in children with reduced renal function. Pediatr Nephrol. 2005;20:1675–86.
National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int suppl 3. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.
Perazella MA. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2012;81:1172–8.
Feinstein JA, Feudther C, Kempe A. Adverse drug event-related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133:e1575–85.
Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, Seid M, Ashby M, Foertmeyer N, Brunner L, Lesko A, Barclay C, Lannon C, Muething S. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013;132:e756–67.
Moffet BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6:856–63.
Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165:522–7.
Goldstein SL, Jaber BL, Faubel S, Chawla LS. Acute Kidney Injury Advisory Group of American Society of Nephrology. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8:476–83.
Goldstein SL, Devarajan P. Acute kidney injury in childhood: should we be worried about progression to CKD? Pediatr Nephrol. 2011;26:509–22.
Askenazi DJ, Feig DI, Graham NM, Huo-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–9.
Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–9.
Ponte B, Felipe C, Muriel A, Tenorio MT, Liano F. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transpl. 2008;23:3859–66.
Surana S, Kumar N, Vasudeva A, Shaikh G, Jhaveri KD, Shah H, Malieckal D, Fogel J, Sidhu G, Rubinstein S. Awareness and knowledge among internal medicine house-staff for dose adjustment of commonly used medications in patients with CKD. BMC Nephrol. 2017;18:26.
Rinke ML, Bundy DG, Velasquez CA, Rao S, Zerhouni Y, Lobner K, Blanck JF, Miller MR. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134:338–60.
Nuckols TK, Smith-Spangler C, Morton SC, Asch SM, Patel VM, Anderson LJ, Deichsel EL, Shekelle PG. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev. 2014;3:56.
Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, Lee R, Mekala A, Song J, Komaroff AL, Bates DW. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–44.
Acknowledgements
We wish to express our deep gratitude to Mr. Seiji Uchikawa of the Tokyo Metropolitan Children’s Medical Center Department of Pharmacology as well as Ms. Masako Tomotsune of the Medical Center’s Clinical Research Support Center for providing advice and guidance from the planning stages of this study. We also wish to thank Tokyo Pediatric Clinical Research Network for the support. The authors wish to thank Mr. James R. Valera, an employee of Tokyo Metropolitan Children’s Medical Center, for providing editorial support in the preparation of this manuscript.
Funding
This survey was performed using research funds provided by the 2013 Tokyo Metropolitan Hospitals’ Clinical Research Fund (Special Research) and the Health and Labour Sciences Research Grants (Clinical Trial on Development of New Drugs and Medical Devices).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kazumoto Iijima has received grants from Daiichi Sankyo Co. Ltd.
Ethical approval
All of the researchers in this study complied with the Helsinki Declaration and the Ethical Guidelines for Epidemiological Studies issued by the Ministry of Health, Labour and Welfare of Japan. With regard to informed consent, explanatory documents were attached to each questionnaire, and the act of mailing back the questionnaires was regarded as an indication of the subjects’ willingness to participate in the survey. We fully protected any personal information by not collecting the subjects’ name, initials, or age. The data obtained were anonymized. Prior to implementation, the study received approval (H25-34) from the ethics committee of Tokyo Metropolitan Children’s Medical Center, this study’s sponsoring institution.
Informed consent
With regard to informed consent, explanatory documents were attached to each questionnaire, and the act of mailing back the questionnaires was regarded as an indication of the subjects’ willingness to participate in the survey.
About this article
Cite this article
Harada, R., Ishikura, K., Shinozuka, S. et al. Ensuring safe drug administration to pediatric patients with renal dysfunction: a multicenter study. Clin Exp Nephrol 22, 938–946 (2018). https://doi.org/10.1007/s10157-018-1537-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1537-7