Abstract
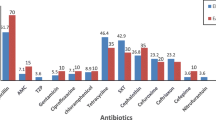
The aim of this study was to investigate the genetic diversity and class 2 integron content of typical and atypical enteropathogenic Escherichia coli (EPEC) strains isolated from children less than 5 years of age. Biochemical tests and serogrou** were performed for identification of isolated strains, and each isolate was tested for susceptibility to 7 antimicrobial agents. The identity of EPEC and their class 2 integron content was confirmed by PCR analysis and sequencing. Subty** of Escherichia coli spp. was performed through pulsed-field gel electrophoresis (PFGE) analysis. All EPEC strains were resistant to 6 antimicrobial agents except for gentamycin. The most prevalent serogroups among EPEC strains were found to be members of O86 and O127 serogroups (37.7 %) and O44, O125, and O128 (42.8 %). The majority of our EPEC isolates (60.7 %) were identified as atypical. Among the total 28 isolates, 4 (14.2 %) harbored a class 2 integron 1,500 or 2,300 bp in size, corresponding to dfrA1–sat1 and dfrA1–sat1–aadA1 resistance gene cassette arrays, respectively. PFGE analysis showed an extensive diversity among the isolates. No PFGE clustering was observed according to bundle-forming pilus (bfp) bacteria, suggesting that PFGE analysis could not discriminate between typical and atypical EPEC strains. The high ratio of antibiotic-resistant strains and the large heterogeneity among EPEC isolates with low prevalence of class 2 integrons signify the need to examine for other mechanism(s) involved in conferring resistance in typical and atypical populations of EPEC.


Similar content being viewed by others
References
Phongpaichit S, Wuttananupan K, Samasanti W. Class 1 integrons and multidrug resistance among Escherichia coli isolates from human stools. Southeast Asian J Trop Med Public Health. 2008;39:279–87.
Chang LL, Chang TM, Chang CY. Variable gene cassette patterns of class 1 integron-associated drug-resistant Escherichia coli in Taiwan. Kaohsiung J Med Sci. 2007;23:273–80.
Alikhani MY, Mirsalehian A, Fatollahzadeh B, Pourshafie MR, Aslani MM. Prevalence of enteropathogenic and shiga toxin-producing Escherichia coli among children with and without diarrhoea in Iran. J Health Popul Nutr. 2007;25:88–93.
Jafari F, Garcia-Gil LJ, Salmanzadeh-Ahrabi S, Shokrzadeh L, Aslani MM, Pourhoseingholi MA, et al. Diagnosis and prevalence of enteropathogenic bacteria in children less than 5 years of age with acute diarrhea in Tehran children’s hospitals. J Infect. 2009;58:21–7.
Soltan Dallal MM, Khorramizadeh MR, MoezArdalan K. Occurrence of enteropathogenic bacteria in children under 5 years with diarrhoea in south Tehran. East Mediterr Health J 2006;12:792–7.
Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508–13.
Ajiboye RM, Solberg OD, Lee BM, et al. Global spread of mobile antimicrobial drug resistance determinants in human and animal Escherichia coli and Salmonella strains causing community-acquired infections. Clin Infect Dis. 2009;49:365–71.
Terajima J, Tamura K, Hirose K, Izumiya H, Miyahara M, Konuma H, et al. A multi-prefectural outbreak of Shigella sonnei infections associated with eating oysters in Japan. Microbiol Immunol. 2004;48:49.
Adabi M, Bakhshi B, Goudarzi H, Zahraei SM, Pourshafie MR. Distribution of class I integron and sulfamethoxazole trimethoprim constin in Vibrio cholerae isolated from patients in Iran. Microb Drug Resist. 2009;15:179–84.
Ahmed AM, Furuta K, Shimomura K, Kasama Y, Shimamoto T. Genetic characterization of multidrug resistance in Shigella spp. from Japan. J Med Microbiol. 2006;55:1685–91.
Goldstein C, Lee MD, Sanchez S, Hudson C, Phillips B, Register B, et al. Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob Agents Chemother. 2001;45:723–6.
Miko A, Pries K, Schroeter A, Helmuth R. Multiple-drug resistance in d-tartrate-positive Salmonella enterica serovar paratyphi B isolates from poultry is mediated by class 2 integrons inserted into the bacterial chromosome. Antimicrob Agents Chemother. 2003;47:3640–3.
McIver CJ, White PA, Jones LA, Karagiannis T, Harkness J, Marriott D, et al. Epidemic strains of Shigella sonnei biotype g carrying integrons. J Clin Microbiol. 2002;40:1538–40.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests; approved standard M2–A8. 8th ed. Wayne: Clinical and Laboratory Standards Institute; 2003.
Najibi S, Bakhshi B, Fallahzad S, Pourshafie MR, Katouli M, Sattari M, et al. Distribution of class 1 integrons among enteropathogenic Escherichia coli. Can J Microbiol. 2012;58:637–43.
Giron JA, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–3.
Blanco M, Blanco JE, Mora A, Rey J, Alonso JM, Hermoso M, et al. Serotypes, virulence genes, and intimin types of shiga toxin (verotoxin)-producing Escherichia coli isolates from healthy sheep in Spain. J Clin Microbiol. 2003;41:1351–6.
White PA, McIver CJ, Rawlinson WD. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob Agents Chemother. 2001;45:2658–61.
Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, et al. Standardization of pulsed-field gel electrophoresis protocols for the subty** of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67.
Kam KM, Luey CK, Tsang YM, Law CP, Chu MY, Cheung TL, et al. Molecular subty** of Vibrio cholerae O1 and O139 by pulsed-field gel electrophoresis in Hong Kong: correlation with epidemiological events from 1994 to 2002. J Clin Microbiol. 2003;41:4502–4511.
Dow MA, Toth I, Malik A, Herpay M, Nógrády N, Ghenghesh KS, et al. Phenotypic and genetic characterization of enteropathogenic Escherichia coli (EPEC) and enteroaggregative E. coli (EAEC) from diarrhoeal and non-diarrhoeal children in Libya. Comp Immunol Microbiol Infect Dis. 2006;29:100–13.
Kobayashi RKT, Saridakis HO, Dias AMG. Molecular identification of enteropathogenic Escherichia coli (EPEC) associated with infant diarrhea in Londrina, Parana, Brazil. Braz J Microbiol. 2000;31:275–80.
Bisi-Johnson MA, Obi CL, Vasaikar SD, Baba KA, Hattori T. Molecular basis of virulence in clinical isolates of Escherichia coli and Salmonella species from a tertiary hospital in the Eastern Cape, South Africa. Gut Pathog. 2011;3:1–9.
Amisano G, Fornasero S, Migliaretti G, Caramello S, Tarasco V, Savino F. Diarrheagenic Escherichia coli in acute gastroenteritis in infants in North-West Italy. New Microbiol 2011;34:45–51.
Albert MJ, Rotimi VO, Dhar R, Silpikurian S, Pacsa AS, Molla AM, et al. Diarrhoeagenic Escherichia coli are not a significant cause of diarrhoea in hospitalised children in Kuwait. BMC Microbiol. 2009;9:62.
Trabulsi LR, Keller R, Tardelli Gomes TAT. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis 2002;8:508–13.
Dawes FE. Antibiotic resistance genes located in integrons isolated from Escherichia coli recovered from humans and animals. Doctor of Philosophy thesis, School of Biological Sciences, University of Wollongong; 2009.
Soto SM, Lobato MJ, Mendoza MC. Class 1 integron-borne gene cassettes in multidrug-resistant Yersinia enterocolitica strains of different phenotypic and genetic types. Antimicrob Agents Chemother. 2006;47:421–5.
Acknowledgments
We thank the Research Council of Tarbiat Modares University for supporting the project.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bakhshi, B., Fallahzad, S. & Pourshafie, M.R. The occurrence of atypical enteropathogenic Escherichia coli strains among children with diarrhea in Iran. J Infect Chemother 19, 615–620 (2013). https://doi.org/10.1007/s10156-012-0526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-012-0526-0