Abstract
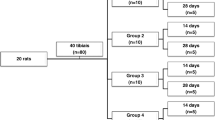
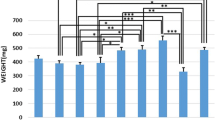
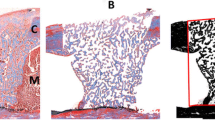
Low-level laser therapy (LLLT) benefits bone metabolism, but its use needs to be standardized. We evaluated the effects of LLLT on bone defects in calvaria of ovariectomized rats. Stereology was used to calculate tissue repair volume (V tr ), density of trabecular bone volume (Vv t ), total volume of newly formed trabecular bone (Vtot), and the area occupied by collagen fibers (A C ). Fifty-four Wistar rats were submitted to bilateral ovariectomy, and bone defects were created in calvaria after 150 days. The animals were divided into nine groups (n = 6), and 24 h after defects, the treatment started with a 780-nm low-intensity GaAlAs laser: G1, G2, and G3 received 3 sessions of 0, 20, and 30 J/cm2 respectively; G4, G5, and G6 received 6 sessions of 0, 20, and 30 J/cm2, respectively; and G7, G8, and G9 received 12 sessions of 0, 20, and 30 J/cm2, respectively. A normal distribution was found for all of the data. The test used to verify the normality was the Kolmogorov-Smirnov (KS, p > 0.05). The one-way ANOVA followed by Tukey’s post hoc test was used for data processing. A difference of p < 0.05 was considered statistically significant. Groups G2 and G1 showed significance for V tr , Vv t , Vtot, and (A C ). Results were significant for (Vv t ) and (Vtot) between G3 and G1. There were no significant results between G5 and G4 as well as between G8 and G7. Groups G6 and G4 results showed statistical difference for V tr , Vv t , Vtot, and (A C ). Groups G9 and G7 showed significance for V tr , Vv t , Vtot, and (A C ). In conclusion, there was new bone formation in the groups that received 20 and 30 J/cm2 when compared to control groups, but over time, the dose of 30 J/cm2 showed better stereological parameters when compared to 20 J/cm2.



Similar content being viewed by others
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Payne JB, Reinhardt RA, Nummikoski PV, Patil KD (1999) Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 10:34–40
Martínez-Maestre MÁ, González-Cejudo C, Machuca G, Torrejón R, Castelo-Branco C (2010) Periodontitis and osteoporosis: a systematic review. Climacteric 13:523–529
Dodd DZ, Rowe DJ (2013) The relationship between postmenopausal osteoporosis and periodontal disease. J Dent Hyg 87:336–344
Pereira FM, Rodrigues VP, Oliveira AE, Brito LM, Lopes FF (2015) Association between periodontal changes and osteoporosis in postmenopausal women. Climacteric 18:311–315
von Wowern N, Klausen B, Kollerup G (1994) Osteoporosis: a risk factor in periodontal disease. J Periodontol 65:1134–1138
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Eduardo CP (2010) Lasers em odontologia. Guanabara Koogan, Rio de Janeiro
Pretel H, Lizarelli RF, Ramalho LT (2007) Effect of low-level laser therapy on bone repair: histological study in rats. Lasers Surg Med 39:788–796
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Lirani-Galvão AP, Jorgetti V, Silva OL (2006) Comparative study of how low-level laser therapy and low-intensity pulsed ultrasound affect bone repair in rats. Photomed Laser Surg 24:735–740
Poppi RR, Silva AL, Nacer RS, Vieira RP, Oliveira LVF, Júnior NSF, Carvalho PTC (2011) Evaluation of the osteogenic effect of low-level laser therapy (808 nm and 660 nm) on bone defects induced in the femurs of female rats submitted to ovariectomy. Lasers Med Sci 26:515–522
Freire MRS, Almeida D, Santos JN, Sarmento VA (2010) The photobiomodulation in the bone repair after radiotherapy: experimental study in rats. Proc. SPIE 7552, Mechanisms for Low-Light Therapy V, 75520H, doi:10.1117/12.842314
Pinheiro ALB, Oliveira MG, Martins PPM, Ramalho LMP, Oliveira MAM, Novaes Júnior A, Nicolau RA (2001) Biomodulatory effects of LLLT on bone regeneration. Laser Ther 13:73–79
Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614
Chicarelli M, Ramos FMM, Manzi FR, Novaes PD, Bóscolo FN, Almeida SM (2007) Effect of gamma rays on the bone repair process in rats with estrogen deficiency. Braz Oral Res 21:75–80
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, de Araújo HS, Renno AC (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29:311–317
Nyengaard JR, Gundersen HJG (1992) The isector: a simple and direct method for generating isotropic, uniform random sections from small pieces. J Microsc 165:427–431
Gundersen HJD (2002) The smooth fractionator. J Microsc 207:191–210
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA (2008) The laboratory rat as an animal model for osteoporosis research. Comp Med 58:424–430
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:171–192
Frost HM, Jee WS (1992) On the rat model of human osteopenias and osteoporosis. Bone Miner 18:227–236
Wronski TJ, Dann LM, Horner SL (1989) Time course of vertebral osteopenia in ovariectomized rats. Bone 10:295–301
Wronski TJ, Dann LM, Scott K, Cintrón M (1989) Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int 45:360–366
Li M, Shen Y, Wronski TJ (1997) Time course of femoral neck osteopenia in ovariectomized rats. Bone 20:55–61
Schimitz JP, Hollinger JO (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 205:299–308
Rawlinson SCF, Boyde A, Davis GR, Howell PGT, Hughes FJ, Kingsmill VJ (2009) Ovariectomy vs. hypofunction: their effects on rat mandibular bone. J Dent Res 88:615–620
Dai Q-G, Zhang P, Wu Y-Q, Ma X-H, Pang J, Jiang L-Y, Fang B (2014) Ovariectomy induces osteoporosis in the maxillary alveolar bone: an in vivo micro-CT and histomorphometric analysis in rats. Oral Dis 20:514–520
Silva AGP (2007) www.aulas.e-agps.info/estereologia/estereologia.pdf. Acessed 26 June 2014
Howard CV, Reed MG (2010) Unbiased stereology. QTP Publications, Liverpool
Karu TI, Lubart R (2000) Effects of low-power light on biological systems. V. SPIE Proc 4159:1–17
Renno AC, Moura FM, Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) Effects of 830-nm laser light on preventing bone loss after ovariectomy. Photomed Laser Surg 24:642–645
Pires-Oliveira DA, Oliveira RF, Amadei SU, Pacheco-Soares C, Rocha RF (2010) Laser 904 nm action on bone repair in rats with osteoporosis. Osteoporos Int 21:2109–2114
Siessere S, Sousa LG, Issa JP, Iyomasa MM, Pitol DL, Barbosa AP, Semprini M, Sebald W, Bentley MV, Regalo SC (2011) Application of low-level laser irradiation (LLLI) and rhBMP-2 in critical bone defect of ovariectomized rats: histomorphometric evaluation. Photomed Laser Surg 29:453–458
Bossini PS, Renno ACM, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz MA, Parizotto NA (2012) Low level laser therapy (830 nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol 47:136–142
Rosa AP, de Sousa LG, Regalo SC, Issa JP, Barbosa AP, Pitol DL, de Oliveira RH, de Vasconcelos PB, Dias FJ, Chimello DT, Siéssere S (2012) Effects of the combination of low-level laser irradiation and recombinant human bone morphogenetic protein-2 in bone repair. Lasers Med Sci 27:971–977
Pinheiro ALB, Gerbi MEMM (2006) Photoengineering of bone repair process. Photomed Laser Surg 24:169–178
Tim CR, Pinto KN, Rossi BR, Fernandes K, Matsumoto MA, Parizotto NA, Rennó CN (2014) Low-level laser therapy enhances the expression of osteogenic factors during bone repairs in rats. Lasers Med Sci 29:146–156
Sella VR, Bomfim FR, Machado PC, Silva Morsoleto MJ, Chohfi M, Plapler H (2015) Effect of low-level laser therapy on bone repair: a randomized controlled experimental study. Lasers Med Sci 30:1061–1068
Theodoro LH, Caiado RC, Longo M, Novaes VC, Zanini NA, Ervolino E, de Almeida JM, Garcia VG (2014) Effectiveness of the diode laser in the treatment of ligature-induced periodontitis in rats: a histopathological, histometric, and immunohistochemical study. Lasers Med Sci Apr 15
Soares LG, Marques AM, Guarda MG, Aciole JM, dos Santos JN, Pinheiro AL (2014) Influence of the λ780nm laser light on the repair of surgical bone defects grafted or not with biphasic synthetic micro-granular hydroxylapatite + beta-calcium triphosphate. J Photochem Photobiol B 131:16–23
Fernandes KR, Ribeiro DA, Rodrigues NC, Tim C, Santos AA, Parizotto NA, Araujo HS, Driusso P, Rennó AC (2013) Effects of low-level laser therapy on the expression of osteogenic genes related in the initial stages of bone defects in rats. J Biomed Opt 18:038002. doi:10.1117/1
Almeida AL, Medeiros IL, Cunha MJ, Sbrana MC, Oliveira PG, Esper LA (2013) The effect of low-level laser on bone healing in critical size defects treated with or without autogenous bone graft: an experimental study in rat calvaria. Clin Oral Implants Res 25:1131–1136. doi:10.1111/clr.12239
Xu M, Deng T, Mo F, Deng B, Lam W, Deng P, Zhang X, Liu S (2009) Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed Laser Surg 27:309–315
Saad A, Yamany ME, Abbas O, Yehia M (2010) Possible role of low level laser therapy on bone turnover in ovariectomized rats. Endocr Regul 44:155–163
Kanenari M, Zhao J, Abiko Y (2011) Enhancement of microtubule-associated protein-1 alpha gene expression in osteoblasts by low level laser irradiation. Laser Ther 20:47–51
Acknowledgments
We thank FAPESP (São Paulo Research Foundation—Processes No. 2011/50686-0 and 2012/10184-9), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and Pró-Reitoria de Pesquisa da Universidade de São Paulo for financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
“All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scalize, P.H., de Sousa, L.G., Regalo, S.C.H. et al. Low-level laser therapy improves bone formation: stereology findings for osteoporosis in rat model. Lasers Med Sci 30, 1599–1607 (2015). https://doi.org/10.1007/s10103-015-1773-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1773-y