Abstract
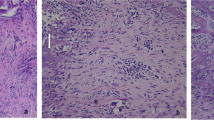
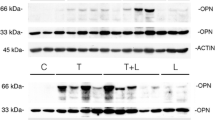
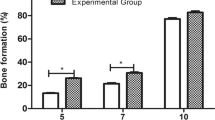
Low-level laser irradiation can promote the healing process in soft and hard tissue but the precise mechanisms are unclear. In this study, we examined the effect of LLLT (low-level laser therapy) on the healing of extraction sockets in diabetic and healthy rats. Forty-eight Sprague-Dawley rats were divided into normal (n = 24) and diabetic (n = 24) rats, and streptozotocin (STZ) injection was used to induce diabetes in the latter. The left and right maxillary first molars of all the rats were extracted. In the non-diabetic rats, the left extraction sockets were not irradiated (group 1) and the right ones were irradiated daily for 3, 5, 7, and 14 days after extraction with a galium-aluminum-arsenide (GaAlAs) diode laser (group 2), and in the diabetic rats, similarly the left ones were not irradiated (group 3) and the right ones were irradiated (group 4). Specimens acquired at these intervals were examined by hematoxylin and eosin (H&E) staining and reverse transcription polymerase chain reaction (RT-PCR). Histological observations and gene expression analyses revealed that groups 2 (normal rats with LLLT) and 4 (diabetic rats with LLLT) showed faster initial healing and more new alveolar bone formation than group 1 (normal rats without LLLT) and group 3 (diabetic rats without LLLT), respectively. We conclude that 980-nm GaAlAs low-intensity diode laser irradiation is beneficial for the initial stages of alveolar bone healing and for further calcification in both diabetic and normal rats when applied every day at a dose of 13.95 J/cm2 for 60 s.


Similar content being viewed by others
References
Yang BK, Lee HC, Lee JY, Son KB, Seol YJ, Lee SC, Kye SB, Chung CP, Han SB (2000) The effect of bioresorbable membrane on the bone regeneration of streptozotocin induced diabetic rats. J Kor Acad Periodontol 30:287–301
Larmey PJ, Darwazeh AM, Frier BM (1982) Oral disorders associated with diabetes mellitus. Diabet Med 9:410–416
Seifter E, Rettura G, Padawer J, Startford E, Kambosos D, Levenson SM (1981) Impaired wound healing in streptozotocin diabetes: prevention by supplement Vit A. Ann Surg 194:42–50
Goodman WG, Horri MT (1984) Diminished bone formation in experimental diabetes. Diabetes 33:825–831
Shin SH, Kim JR, Park BS (2000) Bone formation around titanium implants in the tibiae of streptozotocin-induced diabetic rats. J Kor Maxillofac Plast Rec Surg 22:522–541
Schneir ML, Ramamurthy NS, Golub LM (1984) Extensive degradation of recently synthesized collagen in gingiva of normal and streptozotocin induced diabetic rats. J Dent Res 63:23–27
Nam KY (1984) An experimental study on the healing of extraction wound in diabetic rats. J Kor Oral Maxillofac Surg 10:173–192
Devlin H, Garland H, Sloan P (1996) Healing of tooth extraction sockets in experimental diabetic mellitus. J Oral Maxillofac Surg 54:1087–1091
Maiman TH (1960) Stimulated optic radiation in ruby lasers. Nature 187:493–494
Conlan MJ, Rapley JW, Cobb CM (1996) Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol 23:492–496
Mester E, Spiry T, Szende B, Tota JG (1971) Effects of laser rays on wound healing. Am J Surg 122:532–535
Schindl A, Schind M, Pernerstorfer-Schon H, Schindl L (2000) Low-intensity laser therapy: a review. J Invest Med 48:312–326
Farouk AH (2007) Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed laser Surg 25:72–77
Simões A, Ganzerla E, Yamaguti PM, de Paula EC, Nicolau J (2009) Effect of diode laser on enzymatic activity of parotid glands of diabetic rats. Lasers Med Sci 24:591–596
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG (2008) Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol 79:2156–2165
Lee YJ, Park JB, Kwon YH, Herr Y, Jung JH, Jee YJ, Kang KL (2009) Effect of low-energy laser irradiation on extraction socket healing in streptozotocin-induced diabetic rats. Tissue Eng Regen Medicine 6:568–576
Tu Q, Zhang J, James L, Dickson J, Tang J, Yang P, Chen J (2007) Cbfa1/Runx2-deficiency delays bone wound healing and locally delivered Cbfa1/Runx2 promotes bone repair in animal models. Wound Repair Regen 15:404–412
Lomke MA (2009) Clinical applications of dental lasers. Gen Dent 57:47–59
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofac Orthop 111:525–532
Kawalec JS, Hethenngton VJ, Pfennigwerth TC (2004) Effect of a diode laser on wound healing by using diabetic and nondiabetic mice. J Foot Ankle Surg 43:214–220
Ozawa Y, Shimizu N, Mishima H, Kariya G, Yamaguchi M, Takiguchi H, Iwasawa T, Abiko Y (1995) Stimulatory effects of low power laser irradiation on bone formation in vitro. SPIE 1984:281–288
Coluzzi DJ (2000) An overview of laser wavelengths used in dentistry. Dent Clin North Am 44:753–765
Karu TI, Kalyakov SF (2005) Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 23:355–361
Yamagishi H, Shinohara C, Saito S, Sasaki H, Kanegae H, Shibasaki Y (1994) A basic study on the use of penetrative sensitivity on living tissue. J Jpn Soc Laser Dent 5:13–22
Bossy I, Chevalier JM, Sambuc P (1985) In vitro survey of low energy laser beam penetration in compact bone. Acupunct Electrother Res 10:35–39
Pereira CL, Sallum EA, Nociti FH, Moreira RWF (2009) The effects of low-intensity laser therapy on bone healing around titanium implants: a histometric study in rabbits. Int J Oral Maxillofac Implants 24:47–51
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354
Takeda Y (1988) Irradiation effect of lower-energy laser on alveolar bone after tooth extraction. Int J Oral Maxillofac Surg 17:388–391
Kang KL, Chung JH (2009) Effect of low-energy laser irradiation on extraction sockets in ovariectomized rats. Tissue Eng Regen Medicine 6:1310–1320
Min HJ, Lee MJ, Kim JY, Cho SW, Park HD, Lee SI, Kim HJ, Jung HS (2007) Alteration of BMP-4 and Runx2 expression patterns in mouse temporomandibular joint after ovariectomy. Oral Dis 13:220–227
Götz W, Gerber T, Michel B, Lossdörfer S, Henke KO, Heinemann F (2008) Immunohistochemical characterization of nanocrystalline hydroxiapatite silica gel (NanoBone(s)) osteogenesis: a study on biopsies from human jaws. Clin Oral Implants Res 19:1016–1126
Franceschi RT, Ge C, **ao G, Roca H, Jiang D (2009) Transcriptional regulation of osteoblasts. Cells Tissues Organs 189:144–152
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143:420–430
Tanaka S, Matsuzaka K, Sato D, Inoue T (2007) Characteristics of newly formed bone during guided bone regeneration: analysis of Cbfa-1, osteocalcin, and VEGF expression. J Oral Implantol 33:321–326
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.J., Kang, K.L. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Lasers Med Sci 27, 223–230 (2012). https://doi.org/10.1007/s10103-011-0944-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0944-8