Abstract
Objective
This study aimed to analyze the characteristics of cognitive impairment in adult-onset neuronal intranuclear inclusion disease (NIID).
Methods
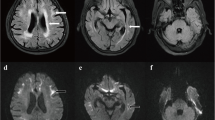
Seven patients with adult-onset NIID were collected consecutively from the memory clinic of Xuanwu hospital from February to December 2019. These cases were diagnosed with skin biopsy triggered by DWI high-intensity signals in corticomedullary junction on brain MRI. We used a battery of neuropsychological scales to detect the patient’s performance in each cognitive domain, and made a detailed analysis on the characteristics of cognitive impairment.
Results
All seven cases had cognitive impairment, and four of them had met the criteria for dementia. The scores of Montreal Cognitive Assessment and Frontal Assessment Battery were abnormal in all patients. The executive dysfunction was confirmed by the abnormal scores of Trail Making Test (5/7, 71%) and Clock Drawing Test (4/7, 57%). Bad performance in Auditory Verbal Learning Test (6/7, 86%) demonstrated that the memory was also a very commonly impaired cognitive domain. The low score on the animal fluency (4/7, 57%), Boston Naming Test (3/7, 43%), and Pentagon and Cube Copying Test (4/7, 57%) indicated that the language and visuospatial skills were also impaired. Fazekas scores were significantly correlated to the global cognition, executive and language functions (r = 0.788–0.906, P < 0.05).
Conclusions
There is obvious impairment in multiple cognitive domains in adult-onset NIID, and both the executive dysfunction and memory deficit are very common. Leukoencephalopathy may be the main course of cognitive impairment in adult-onset NIID.



Similar content being viewed by others
References
Takahashi-Fujigasaki J (2003) Neuronal intranuclear hyaline inclusion disease. Neuropathology 23(4):351–359. https://doi.org/10.1046/j.1440-1789.2003.00524.x
Sone J, Mori K, Inagaki T, Katsumata R, Takagi S, Yokoi S, Araki K, Kato T, Nakamura T, Koike H, Takashima H, Hashiguchi A, Kohno Y, Kurashige T, Kuriyama M, Takiyama Y, Tsuchiya M, Kitagawa N, Kawamoto M, Yoshimura H, Suto Y, Nakayasu H, Uehara N, Sugiyama H, Takahashi M, Kokubun N, Konno T, Katsuno M, Tanaka F, Iwasaki Y, Yoshida M, Sobue G (2016) Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 139(Pt 12):3170–3186. https://doi.org/10.1093/brain/aww249
Raza HK, Singh S, Rai P, Chansysouphanthong T, Amir A, Cui G, Song W, Bao L, Zhou S, Shi H, Chen H (2020) Recent progress in neuronal intranuclear inclusion disease: a review of the literature. Neurol Sci 41(5):1019–1025. https://doi.org/10.1007/s10072-019-04195-6
Tian Y, Wang JL, Huang W, Zeng S, Jiao B, Liu Z, Chen Z, Li Y, Wang Y, Min HX, Wang XJ, You Y, Zhang RX, Chen XY, Yi F, Zhou YF, Long HY, Zhou CJ, Hou X, Wang JP, **e B, Liang F, Yang ZY, Sun QY, Allen EG, Shafik AM, Kong HE, Guo JF, Yan XX, Hu ZM, **a K, Jiang H, Xu HW, Duan RH, ** P, Tang BS, Shen L (2019) Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet 105(1):166–176. https://doi.org/10.1016/j.ajhg.2019.05.013
Sone J, Mitsuhashi S, Fujita A, Mizuguchi T, Hamanaka K, Mori K, Koike H, Hashiguchi A, Takashima H, Sugiyama H, Kohno Y, Takiyama Y, Maeda K, Doi H, Koyano S, Takeuchi H, Kawamoto M, Kohara N, Ando T, Ieda T, Kita Y, Kokubun N, Tsuboi Y, Katoh K, Kino Y, Katsuno M, Iwasaki Y, Yoshida M, Tanaka F, Suzuki IK, Frith MC, Matsumoto N, Sobue G (2019) Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet 51(8):1215–1221. https://doi.org/10.1038/s41588-019-0459-y
Deng J, Gu M, Miao Y, Yao S, Zhu M, Fang P, Yu X, Li P, Su Y, Huang J, Zhang J, Yu J, Li F, Bai J, Sun W, Huang Y, Yuan Y, Hong D, Wang Z (2019) Long-read sequencing identified repeat expansions in the 5’UTR of the NOTCH2NLC gene from Chinese patients with neuronal intranuclear inclusion disease. J Med Genet 56(11):758–764. https://doi.org/10.1136/jmedgenet-2019-106268
Sone J, Hishikawa N, Koike H, Hattori N, Hirayama M, Nagamatsu M, Yamamoto M, Tanaka F, Yoshida M, Hashizume Y, Imamura H, Yamada E, Sobue G (2005) Neuronal intranuclear hyaline inclusion disease showing motor-sensory and autonomic neuropathy. Neurology 65(10):1538–1543. https://doi.org/10.1212/01.wnl.0000184490.22527.90
Nakamura M, Ueki S, Kubo M, Yagi H, Sasaki R, Okada Y, Akiguchi I, Kusaka H, Kondo T (2018) Two cases of sporadic adult-onset neuronal intranuclear inclusion disease preceded by urinary disturbance for many years. J Neurol Sci 392:89–93. https://doi.org/10.1016/j.jns.2018.07.012
Fujita K, Osaki Y, Miyamoto R, Shimatani Y, Abe T, Sumikura H, Murayama S, Izumi Y, Kaji R (2017) Neurologic attack and dynamic perfusion abnormality in neuronal intranuclear inclusion disease. Neurol Clin Pract 7(6):e39–e42. https://doi.org/10.1212/CPJ.0000000000000389
Liu Y, Mimuro M, Yoshida M, Hashizume Y, Niwa H, Miyao S, Ujihira N, Akatsu H (2008) Inclusion-positive cell types in adult-onset intranuclear inclusion body disease: implications for clinical diagnosis. Acta Neuropathol 116(6):615–623. https://doi.org/10.1007/s00401-008-0442-7
Sone J, Tanaka F, Koike H, Inukai A, Katsuno M, Yoshida M, Watanabe H, Sobue G (2011) Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology 76(16):1372–1376. https://doi.org/10.1212/WNL.0b013e3182166e13
Araki K, Sone J, Fujioka Y, Masuda M, Ohdake R, Tanaka Y, Nakamura T, Watanabe H, Sobue G (2016) Memory loss and frontal cognitive dysfunction in a patient with adult-onset neuronal intranuclear inclusion disease. Intern Med 55(16):2281–2284. https://doi.org/10.2169/internalmedicine.55.5544
Wang Y, Wang B, Wang L, Yao S, Zhao J, Zhong S, Cong L, Liu L, Zhang J, Zhang J, Hong D (2020) Diagnostic indicators for adult-onset neuronal intranuclear inclusion disease. Clin Neuropathol 39(1):7–18. https://doi.org/10.5414/NP301203
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van Gool WA (1995) Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol 242(9):557–560. https://doi.org/10.1007/BF00868807
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3):183–194. https://doi.org/10.1111/j.1365-2796.2004.01388.x
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC (2004) Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256(3):240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (IV-TR), 4th —text revised. Washington, DC
Takahashi-Fujigasaki J, Nakano Y, Uchino A, Murayama S (2016) Adult-onset neuronal intranuclear hyaline inclusion disease is not rare in older adults. Geriatr Gerontol Int 16(Suppl 1):51–56. https://doi.org/10.1111/ggi.12725
Sone J, Kitagawa N, Sugawara E, Iguchi M, Nakamura R, Koike H, Iwasaki Y, Yoshida M, Takahashi T, Chiba S, Katsuno M, Tanaka F, Sobue G (2014) Neuronal intranuclear inclusion disease cases with leukoencephalopathy diagnosed via skin biopsy. J Neurol Neurosurg Psychiatry 85(3):354–356. https://doi.org/10.1136/jnnp-2013-306084
Yokoi S, Yasui K, Hasegawa Y, Niwa K, Noguchi Y, Tsuzuki T, Mimuro M, Sone J, Watanabe H, Katsuno M, Yoshida M, Sobue G (2016) Pathological background of subcortical hyperintensities on diffusion-weighted images in a case of neuronal intranuclear inclusion disease. Clin Neuropathol 35(6):375–380. https://doi.org/10.5414/NP300961
Takahashi J, Fukuda T, Tanaka J, Minamitani M, Fujigasaki H, Uchihara T (2000) Neuronal intranuclear hyaline inclusion disease with polyglutamine-immunoreactive inclusions. Acta Neuropathol 99(5):589–594. https://doi.org/10.1007/s004010051166
Sloane AE, Becker LE, Ang LC, Wark J, Haslam RH (1994) Neuronal intranuclear hyaline inclusion disease with progressive cerebellar ataxia. Pediatr Neurol 10(1):61–66. https://doi.org/10.1016/0887-8994(94)90070-1
Schmahmann JD, Smith EE, Eichler FS, Filley CM (2008) Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309. https://doi.org/10.1196/annals.1444.017
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG (2009) Cadasil. Lancet Neurol 8(7):643–653. https://doi.org/10.1016/S1474-4422(09)70127-9
Jung WB, Mun CW, Kim YH, Park JM, Lee BD, Lee YM, Moon E, Jeong HJ, Chung YI (2014) Cortical atrophy, reduced integrity of white matter and cognitive impairment in subcortical vascular dementia of Binswanger type. Psychiatry Clin Neurosci 68(12):821–832. https://doi.org/10.1111/pcn.12196
Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM (2005) Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 128(Pt 9):2034–2041. https://doi.org/10.1093/brain/awh553
Abe K, Fujita M (2017, 2017) Over 10 years MRI observation of a patient with neuronal intranuclear inclusion disease. BMJ Case Rep. https://doi.org/10.1136/bcr-2016-218790
Kanno S, Abe N, Saito M, Takagi M, Nishio Y, Hayashi A, Uchiyama M, Hanaki R, Kikuchi H, Hiraoka K, Yamasaki H, Iizuka O, Takeda A, Itoyama Y, Takahashi S, Mori E (2011) White matter involvement in idiopathic normal pressure hydrocephalus: a voxel-based diffusion tensor imaging study. J Neurol 258(11):1949–1957. https://doi.org/10.1007/s00415-011-6038-5
Filley CM (2012) White matter dementia. Ther Adv Neurol Disord 5(5):267–277. https://doi.org/10.1177/1756285612454323
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, Zhou L, Martins R, Ellis KA, Masters CL, Ames D, Salvado O, Rowe CC (2013) Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol 70(7):903–911. https://doi.org/10.1001/jamaneurol.2013.1062
Takahashi J, Tanaka J, Arai K, Funata N, Hattori T, Fukuda T, Fujigasaki H, Uchihara T (2001) Recruitment of nonexpanded polyglutamine proteins to intranuclear aggregates in neuronal intranuclear hyaline inclusion disease. J Neuropathol Exp Neurol 60(4):369–376. https://doi.org/10.1093/jnen/60.4.369
Liu Y, Lu J, Li K, Zhao H, Feng Y, Zhang Z, Hu L, Li G, Shao Y, Wang Y (2019) A multimodal imaging features of the brain in adult-onset neuronal intranuclear inclusion disease. Neurol Sci 40(7):1495–1497. https://doi.org/10.1007/s10072-019-03742-5
Gelpi E, Botta-Orfila T, Bodi L, Marti S, Kovacs G, Grau-Rivera O, Lozano M, Sanchez-Valle R, Munoz E, Valldeoriola F, Pagonabarraga J, Tartaglia GG, Mila M (2017) Neuronal intranuclear (hyaline) inclusion disease and fragile X-associated tremor/ataxia syndrome: a morphological and molecular dilemma. Brain 140(8):e51. https://doi.org/10.1093/brain/awx156
Sugiyama A, Sato N, Kimura Y, Maekawa T, Enokizono M, Saito Y, Takahashi Y, Matsuda H, Kuwabara S (2017) MR imaging features of the cerebellum in adult-onset neuronal intranuclear inclusion disease: 8 cases. AJNR Am J Neuroradiol 38(11):2100–2104. https://doi.org/10.3174/ajnr.A5336
Acknowledgments
The authors want to thank all the patients and participants involved in the study.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81100797) and Bei**g high-level health talents training project (No. 2015-3-068).
Author information
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the regional clinical research ethics committee.
Informed consent
All participants gave written informed consent prior to their participation in the study, in accordance with the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Wang, F., Ma, X., Shi, Y. et al. Cognitive profiles in adult-onset neuronal intranuclear inclusion disease: a case series from the memory clinic. Neurol Sci 42, 2487–2495 (2021). https://doi.org/10.1007/s10072-020-04864-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04864-x