Abstract
Background and objective
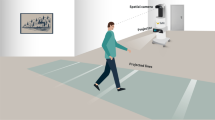
In a proof-of-concept study, we aimed to verify whether the wearable haptic anklets, a device that delivers personalized suprathreshold alternating exteroceptive stimulation at the anklets on demand, may improve the quality of walking, including the freezing of gate (FOG), in idiopathic Parkinson’s disease (PD) patients. The clinical relevance of the presented device as a walking pacemaker to compensate the disturbed locomotion through the generation of a more physiological internal walking rhythm should be verified in a dedicated clinical trial.
Methods
We tested 15 patients diagnosed as idiopathic PD, during their regular treatment regimen. Patients were evaluated during walking with the device switched on and off, personalized at their most comfortable cadence. Stride velocity, variance, and length, as well as FOG episode duration during walking or turning of 180°, were quantified by an optical high-performance motion capture VICON system.
Results
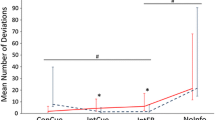
The alternating, rhythmic, sensory stimulation significantly improved either walking velocity or reduced inter-stride variance. Effects were more variable on stride length. The significant reduction of FOG episodes’ duration correlated with clinical severity of scales rating gate and balance. No safety problems occurred.
Conclusions
The WEARHAP-PD device, whose Technology Readiness Level (TRL) is 6, significantly improved some walking abilities (walking velocity and stride variance) and reduced the duration of FOG episodes in idiopathic PD patients. Unlike the traditional auditory and visual explicit cues that require the user’s allocation of attention for correct functioning, the interaction of the patients with the surrounding environment was preserved, due to the likely implicit processing of haptic stimuli.



Similar content being viewed by others
References
Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E (2001) Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord 16:1066–1075
Nonnekes J, Snijders AH, Nutt JG, Deuscl G, Giladi N, Bloem BR (2015) Freezing of gait: a practical approach to management. Lancet Neurol 14:768–778
Abboud H, Genc G, Thompson NR, Oravivattanakul S, Alsallom F, Reyes D, Wilson K, Cerejo R, Yu XX, Floden D, Ahmed A, Gostkowski M, Ezzeldin A, Marouf H, Mansour OY, Machado A, Fernandez HH (2017) Predictors of functional and quality of life outcomes following deep brain stimulation surgery in parkinson's disease patients: disease, patient, and surgical factors. Parkinsons Dis 5609163
Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ (2008) Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology 70:2241–2247
Spaulding SJ, Barber B, Colby M, Cormack B, Mick T, Jenkins ME (2013) Cueing and gait improvement among people with Parkinson’s disease: a meta-analysis. Arch Phys Med Rehabil 94:562–570
Ginis P, Nackaerts E, Nieuwboer A, Heremans E (2018) Cueing for people with Parkinson’s disease with freezing of gait: a narrative review of the state-of-the-art and novel perspectives. Ann Phys Rehabil Med 61:407–413
Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM (1996) Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov Disord 11:193–200
Cochen Decock V, Dotov D, Damm L, Picot MC, Ihalainen P, Driss V, Geny C, Bardy B, Dalla Bella S. (2019) BeatPark: a new wearable device for gait auto-rehabilitation in Parkinson’s disease delivering adapted musical stimulation. Mov Disord 34: (suppl 2) [abstract]
Novak P, Novak V (2006) Effect of step-synchronized vibration stimulation of soles on gait in Parkinson’s disease: a pilot study. J Neuroeng Rehabil 3:9
King LK, Almeida QJ, Ahonen H (2009) Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. NeuroRehabilitation 25:297–306
De Nunzio AM, Grasso M, Nardone A, Godi M, Schieppati M (2010) Alternate rhythmic vibratory stimulation of trunk muscles affects walking cadence and velocity in Parkinson’s disease. Clin Neurophysiol 121:240–247
Winfree KN, Pretzer-Aboff I, Hilgart D, Aggarwal R, Behari M, Agrawal SK (2013) The effect of step-synchronized vibration on patients with Parkinson’s disease: case studies on subjects with freezing of gait or an implanted deep brain stimulator. IEEE Trans Neural Syst Rehabil Eng 5:806–811
Kuo AD (2002) The relative role of feedforward and feedback in the control of rhythmic movements. Mot Control 6:129–145
Dimitrijevic MR, Gerasimenko Y, Pinter MM (1998) Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 860:360–376
Lisini Baldi T, Paolocci G, Prattichizzo D (2018) Human guidance: suggesting walking pace under manual and cognitive load. In EuroHaptics 2018: Haptics: science, technology, and applications, volume 10894, Pages 416–427, Pisa, Italy
Lisini Bladi T, Paolocci G, Barcelli D, Prattichizzo D (2020) Wearable haptics for remote social walking. IEEE Trans Haptics, 2020 (in press)
Scheggi S, Talarico A, Prattichizzo D (2014) A remote guidance system for blind and visually impaired people via vibrotactile haptic feedback. In 22nd Mediterranean conference on control and automation (pp. 20–23). IEEE
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society UPDRS Revision Task Force (2008) Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Barthel C, Nonnekes J, van Helvert M, Haan R, Janssen A, Delval A, Weerdesteyn V, Debû B, van Wezel R, Bloem BR, Ferraye MU (2018) The laser shoes. A new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology 90:e164–e171
Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30:459–463
Tinetti ME, Williams TF, Mayewski R (1986) Tinetti balance assessment tool. Fall risk index for elderly patients based on number of chronic dis- abilities. Am J Med 80:429–434
Gemperle F, Hirsch T, Goode A, Pearce J, Siewiorek D, Smailigic A (2003) “Wearable vibro-tactile display,” Corneige Mellon University
Riener A (2011) Sensor-actuator supported implicit interaction in driver assistance systems, S. H. Et al., Ed. Springer, vol. 10
Roerdink M, Bank PJ, Peper CL, Beek PJ (2011) Walking to the beat of different drums: practical implications for the use of acoustic rhythms in gait rehabilitation. Gait Posture 33:690–694
Ahn J, Hogan N (2012) Walking is not like reaching: evidence from periodic mechanical perturbations. PLoS One 7:e31767
Leman M, Moelants D, Varewyck M, Styns F, van Noorden L, Martens JP (2013) Activating and relaxing music entrains the speed of beat synchronized walking. PLoS One 8:e67932
Damm L, Varoqui D, De Cock VC, Dalla Bella S, Bardy B (2019) Why do we move to the beat? A multi-scale approach, from physical principles to brain dynamics. Neurosci Biobehav Rev 112:553–584. https://doi.org/10.1016/j.neubiorev.2019.12.024
Abbruzzese G, Berardelli A (2003) Sensorimotor integration in movement disorders. Mov Disord 18:231–240
Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M (2017) Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 378:204–209
Zehr EP, Duysens J (2004) Regulation of arm and leg movement during human locomotion. Neuroscientist 10:347–361
Nielsen JB (2003) How we walk: central control of muscle activity during human walking. Neuroscientist 9:195–204
Kotz SA, Schwartze M (2010) Cortical speech processing unplugged: a timely subcortico-cortical framework. Trends Cogn Sci 14:392–399
Kotz SA, Schwartze M (2011) Differential input of the supplementary motor area to a dedicated temporal processing network: functional and clinical implications. Front Integr Neurosci 5:86
Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10:734–744
Boecker H, Ceballos-Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, Munz F, Schwaiger M, Conrad B (1999) Sensory processing in Parkinson’s and Huntington’s disease: investigations with 3D H(2)(15)O-PET. Brain 122:1651–1665
Ulivelli M, Rossi S, Pasqualetti P, Rossini PM, Ghiglieri O, Passero S, Battistini N (1999) Time course of frontal somatosensory evoked potentials relation to L-dopa plasma levels and motor performance in PD. Neurology 53:1451–1457
Lewis GN, Byblow WD (2002) Altered sensorimotor integration in Parkinson’s disease. Brain 125:2089–2099
Fasano A, Herman T, Tessitore A, Strafella AP, Bohnen NI (2015) Neuroimaging of freezing of gait. J Park Dis 5:241–254
Shik ML, Severin FV, Orlovsky GN (1996) Control of walking and running by means of electrical stimulation of the mid-brain. Biophysics 11:756–765
Chung SJ, Lee YH, Yoo HS, Oh JS, Kim JS, Ye BS, Sohn YH, Lee PH (2019) White matter hyperintensities as a predictor of freezing of gate in Parkinon’s disease. Parkinsonism Relat Disord 66:105–109
Nonnekes J, Ružicka E, Nieuwboer A, Hallett M, Fasano A, Bloem BR (2019) Comopensation strategies for gait impairment in Parkinson diseae. JAMA Neurol 76:718–725
Conte A, Khan N, Defazio G, Rothwell JC, Berardelli A (2013) Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat Rev Neurol 9:687–697
Konczak J, Sciutti A, Avanzino L, Squeri V, Gori M, Masia L, Abbruzzese G, Sandini G (2012) Parkinson’s disease accelerates age-related decline in haptic perception by altering somatosensory integration. Brain 135:3371–3379
Funding
The research leading to these results has received funding from the European Union’s Horizon 2020 research and innovation program—Societal Challenge 1 (DG CONNECT/H) under grant agreement n. 643644 of the project “ACANTO: A CyberphysicAl social NeTwOrk using robot friends” and under grant agreement n° 601165 of the project ``WEARHAP–WEARable HAPtics for humans and robots”.
Author information
Authors and Affiliations
Contributions
Simone Rossi: Study conception, patients’ recruitment, supervision of data acquisition, and manuscript preparation.
Tommaso Lisini Baldi: Device conception and manufacturing, data acquisition and analysis, and manuscript preparation.
Marco Aggravi: Device conception and manufacturing and data acquisition and analysis.
Monica Ulivelli: Patients’ recruitment, clinical coordination, and critical revision of the manuscript.
David Cioncoloni: Data acquisition and organization.
Viola Niccolini: Data acquisition and analysis.
Lorenzo Donati: Data acquisition and analysis.
Domenico Prattichizzo: Device and study conception and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossi, S., Lisini Baldi, T., Aggravi, M. et al. Wearable haptic anklets for gait and freezing improvement in Parkinson’s disease: a proof-of-concept study. Neurol Sci 41, 3643–3651 (2020). https://doi.org/10.1007/s10072-020-04485-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04485-4