Abstract
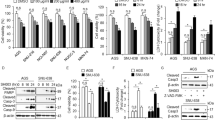
Quercetin has been reported to possess therapeutic effects in the treatment of cancers. In this study, the molecular action of quercetin, with emphasis on its ability to control the intracellular signaling cascades of hypoxia inducible factor-1α (HIF-1α), mammalian target of rapamycin (mTOR), and AMP-activated protein kinase (AMPK), responsible for survival or induction of apoptosis in hypoxic MCF-7 cells, was investigated. The effects of quercetin on apoptosis in relation to its ability to prevent HIF-1α induction were investigated. The involvement of HIF-1α reduction in quercetin-based cancer control was clearly shown in conditions of mTOR inhibition by rapamycin, an mTOR inhibitor. Surprisingly, quercetin induced an AMPK-suppressed state in a CoCl2-induced hypoxic condition. It is speculated that quercetin is capable of inhibiting AMPK to decrease HIF-1α, which is a critical survival factor in hypoxia. A complex control of HIF-1α, mTOR, and AMPK is necessary in inducing apoptosis of MCF-7 breast cancer cells under hypoxia.
Similar content being viewed by others
References
Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 6: 457–470 (2010)
Shaw R. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. 196: 65–80 (2009)
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist. Update 11: 32–50 (2008)
Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 574: 63–71 (2006)
Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol. 196: 55–63 (2009)
Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ, Park OJ. Curcumin exerts antidifferentiation effect through AMPKα-PPAR-γ in 3T3-L1 adipocytes and antiproliferatory effect through AMPKα-COX-2 in cancer cells. J. Agr. Food Chem. 57: 305–310 (2009)
Rutter GA, Da Silva Xavier G, Leclerc I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 375: 1–16 (2003)
Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell Biol. 26: 5336–5347 (2006)
Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J. Physiol. 574: 7–15 (2006)
Axelson H, Fredlund E, Ovenberger M, Landberg G, Påhlman S. Hypoxia-induced dedifferentiation of tumor cells—a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin. Cell Dev. Biol. 16: 554–563 (2005)
Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441: 437–443 (2006)
Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist 5: 10–17 (2004)
Nagle DG, Zhou YD. Natural product-based inhibitors of hypoxiainducible factor-1 (HIF-1). Curr. Drug Targets 7: 355–369 (2006)
Manolescu B, Oprea E, Busu C, Cercasov C. Natural compounds and the hypoxia-inducible factor (HIF) signalling pathway. Biochimie 91: 1347–1358 (2009)
Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, Park H, Kim SS, Choe W, Kang I, Ha J. Reactive oxygen species stabilize hypoxia-inducible factor-1α protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis 29: 713–721 (2008)
Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL, Vera DR, Shaw RJ. mTOR and HIF-1α-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. P. Natl. Acad. Sci. USA 106: 11137–11142 (2009)
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell Biol. 22: 7004–7014 (2002)
Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J. Mol. Med. 85: 1301–1307 (2007)
Semenza GL. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 8: 588–594 (1998)
Brahimi-Horn C, Mazure N, Pouysségur J. Signalling via the hypoxia-inducible factor-1α requires multiple posttranslational modifications. Cell Signal 1: 1–9 (2005)
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. P. Natl. Acad. Sci. USA 92: 5510–5514 (1995)
Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 545: 1337–1340 (2001)
Lee DH, Lee YJ. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1α (HIF-1α) through inhibiting protein synthesis. J. Cell Biochem. 105: 546–553 (2008)
Park SS, Bae I, Lee YJ. Flavonoids-induced accumulation of hypoxia-inducible factor (HIF)-1α/2α is mediated through chelation of iron. J. Cell Biochem. 103: 1989–1998 (2008)
Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePPinho RAN, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 6: 91–99 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, YK., Park, O.J. Involvement of AMPK/mTOR/HIF-1α in anticancer control of quercetin in hypoxic MCF-7 cells. Food Sci Biotechnol 20, 371–375 (2011). https://doi.org/10.1007/s10068-011-0052-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-011-0052-3