Abstract
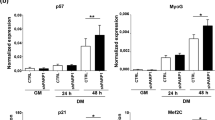
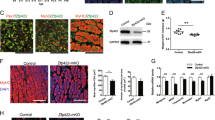
MyoD and myogenin (Myog) recognize sets of distinct but overlap** target genes and play different roles in skeletal muscle differentiation. MyoD is sufficient for near-full expression of early targets, while Myog can only partially enhance expression of MyoD-initiated late muscle genes. However, the way in which Myog enhances the expression of MyoD-initiated late muscle genes remains unclear. Here, we examine the effects of Myog on chromatin remodeling at late muscle gene promoters and their activation within chromatin environment. Chromatin immunoprecipitation (ChIP) assay showed that Myog selectively bound to the regulatory sequences of late muscle genes. Overexpression of Myog was found to overcome sodium butyrateinhibited chromatin at late muscle genes in differentiating C2C12 myoblasts, shifting the transcriptional activation of these genes to an earlier time period. Furthermore, overexpression of Myog led to increased hyperacetylation of core histone H4 in differentiating C2C12 myoblasts but not NIH3T3 fibroblasts, and hyperacetylated H4 was associated directly with the late muscle genes in differentiating C2C12, indicating that Myog can induce chromatin remodeling in the presence of MyoD. In addition, co-immunoprecipitation (CoIP) revealed that Myog was associated with the nuclear protein Brd4 in differentiating C2C12 myoblasts. Together, these results suggest that Myog enhances the expression of MyoD-initiated late muscle genes through MyoD-dependent ability of Myog to induce chromatin remodeling, in which Myog-Brd4 interaction may be involved.
Similar content being viewed by others
References
Ai, N.P., Hu, X.M., Ding, F., Yu, B.F., Wang, H.P., Lu, X.D., Zhang, K., Li, Y.N., Han, A.D., Lin, W., et al. (2011). Signal-induced Brd4 release from chromatin is essential for its role transition from chromatin targeting to transcriptional regulation. Nucleic Acids Res. 39, 9592–9604.
Asakura, A., Fujisawa-Sehara, A., Komiya, T., Nabeshima, Y., and Nabeshima, Y. (1993). MyoD and myogenin act on the chicken myosin light chain 1 gene as distinct transcriptional factors. Mol. Cell. Biol. 13, 7153–7162.
Bergstrom, D.A., Penn, B.H., Strand, A., Perry, R.L., Rudnicki, M.A., and Tapscott, S.J. (2002). Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9, 587–600.
Berkes, C.A., and Tapscott, S.J. (2005). MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16, 585–595.
Berkes, C.A., Bergstrom, D.A., Penn, B.H., Seaver, K.J., Knoepfler, P.S., and Tapscott, S.J. (2004). Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell 14, 465–477.
Blackwell, T.K., and Weintraub, H. (1990). Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science 250, 1104–1110.
Blais, A., Tsikitis, M., Acosta-Alvear, D., Sharan, R., Kluger, Y., and Dynlacht, B.D. (2005). An initial blueprint for myogenic differenttiation. Genes Dev. 19, 553–569.
Braun, T., Buschhausen-Denkerm G., Bober, E., Tannich, E., and Arnold, H.H. (1989). A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 8, 701–709.
Brennan, T.J., Edmondson, D.G., and Olson, E.N. (1990). Aberrant regulation of MyoD1 contributes to the partially defective myogenic phenotype of the BC3H1 muscle cell line. J. Cell Biol. 110, 929–937.
Buckingham, M. (2001). Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 11, 440–448.
Cao, Y., Kumar, R.M., Penn, B.H., Berkes, C.A., Kooperberg, C., Boyer, L.A., Young, R.A., and Tapscott, S.J. (2006). Global and genespecific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J. 25, 502–511.
Covault, J., Sealy, L., Schnell, R., Shires, A., and Chalkley, R. (1982). Histone hypoacetylation following release of HTC cells from butyrate. J. Biol. Chem. 257, 5809–5815.
de la Serna, I.L., Ohkawa, Y., Berkes, C.A., Bergstrom, D.A., Dacwag, C.S., Tapscott, S.J., and Imbalzano, A.N. (2005). MyoD targets chromatin complexes to the myogenin locus prior to forming a stable DNA bound complex. Mol. Cell. Biol. 25, 3997–4009.
Dey, A., Chitsaz, F., Abbasi, A., Misteli, T., and Ozato, K. (2003). The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100, 8758–8763.
Dilworth, F.J., Seaver, K.J., Fishburn, A.L., Htet, S.L., and Tapscott, S.J. (2004). In vitro transcription system delineates the distinct roles of the coactivators pCAF and p300 during MyoD/E47-dependent transactivation. Proc. Natl. Acad. Sci. USA 101, 11593–11598.
Edmondson, D.G., and Olson, E.N. (1989). A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 3, 628–640.
Edmondson, D.G., Cheng, T.C., Cserjesi, P., Chakraborty, T., and Olson, E.N. (1992). Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol. Cell. Biol. 12, 3665–3677.
Gerber, A.N., Klesert, T.R., Bergstrom, D.A., and Tapscott, S.J. (1997). Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 11, 436–450.
Gong, M., Ni, J.H., and Jia, H.T. (2002). Increased exchange rate of histone H1 on chromatin by exogenous myogenin expression. Cell Res. 12, 395–400.
Hasty, P., Bradley, A., Morris, J.H., Edmondson, D.G., Venuti, J.M., Olson, E.N., and Klein, W.H. (1993). Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364, 501–506.
Hatoum, O.A., Gross-Mesilaty, S., Breitschopf, K., Hoffman, A., Gonen, H., Ciechanover, A., and Bengal, E. (1998). Degradation of the myogenic transcription factor MyoD by the ubiquitin pathway in vivo and in vitro: regulation by specific DNA binding. Mol. Cell. Biol. 18, 5670–5677.
Jang, M.K., Mochizuki, K., Zhou, M., Jeong, H.S., Brady, J.N., and Ozato, K. (2005). The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534.
Ji, Z.X., Du, C., Wu, G.S., Li, S.Y., An, G.S., Yang, Y.X., Jia, R., Jia, H.T., and Ni, J.H. (2009). Synergistic up-regulation of muscle LIM protein expression in C2C12 and NIH3T3 cells by myogenin and MEF2C. Mol. Genet. Genomics 281, 1–10.
Johnston, L.A., Tapscott, S.J., and Eisen, H. (1992). Sodium butyrate inhibits myogenesis by interfering with the transcriptional activation function of MyoD and myogenin. Mol. Cell. Biol. 12, 5123–5130.
Kassar-Duchossoy, L., Gayraud-Morel, B., Gomes, D., Rocancourt, D., Buckingham, M., Shinin, V., and Tajbakhsh, S. (2004). Mrf4 determines skeletal muscle identitiy in Myf5: MyoD doublemutant mice. Nature 431, 466–471.
Kitzmann, M., Carnac, G., Vandromme, M., Primig, M., Lamb, N.J. C., and Fernandez, A. (1998). The muscle regulatory factors MyoD and Myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 142, 1447–1459.
Lu, P.Y., Taylor, M., Jia, H.T., and Ni, J.H. (2004). Muscle LIM protein promotes expression of the acetylcholine receptor Γ-subunit gene cooperatively with the myogenin-E12 complex. Cell. Mol. Life Sci. 61, 2386–2392.
Nabeshima, Y., Hanaoka, K., Hayasaka, M., Esuml, E., Li, S., Nonaka, I., and Nabeshima, Y. (1993). Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364, 532–535.
Nojima, M., Huang, Y., Tyagi, M., Kao, H.Y., and Fu**aga, K. (2008). The positive transcription elongation factor b is an essential cofactor for the activation of transcription by myocyte enhancer factor 2. J. Mol. Biol. 382, 275–287.
Ohkawa, Y., Marfella, C.G., and Imbalzano, A.N. (2006). Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brg1. EMBO J. 25, 490–501.
Olson, E.N., and Klein, W.H. (1994). bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 8, 1–8.
Penn, B.H., Bergstrom, D.A., Dilworth, F.J., Bengal, E., and Tapscott, S.J. (2004). A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 18, 2348–2353.
Peterlin, B.M., and Price, D.H. (2006). Controlling the elongation phase of transcription with P-TEFb. Mol. Cell 23, 297–305.
Plesko, M.M., Hargrove, J.L., Granner, D.K., and Chalkley, R. (1983). Inhibition by sodium butyrate of enzyme induction by glucocorticoids and dibutyryl cyclic AMP. J. Biol. Chem. 258, 13738–13744.
Rahl, P.B., Lin, C.Y., Seila, A.C., Flynn, R.A., McCuine, S., Burge, C.B., Sharp, P.A., and Young, R.A. (2010). c-Myc regulates transcriptional pause release. Cell 141, 432–445.
Rahman, S., Sowa, M.E., Ottinger, M., Smith, J.A., Shi, Y., Harper, J.W., and Howley, P.M. (2011). The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol. Cell. Biol. 31, 2641–2652.
Rawls, A., Morris, J.H., Rudnicki, M., Braun, T., Arnold, H.H., Klein, W.H., and Olson, E.N. (1995). Myogenin’s functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev. Biol. 172, 37–50.
Rudnicki, M.A., Schnegelsberg, P.N., Stead, R.H., Braun, T., Arnold, H.H., and Jaenisch, R. (1993). MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351–1359.
Saunders, A., Core, L.J., and Lis, J.T. (2006). Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell. Biol. 7, 557–567.
Simone, C., Forcales, S.V., Hill, D.A., Imbalzano, A.N., Latella, L., and Puri, P.L. (2004) p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36, 738–743.
Tapscott, S.J. (2005). The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132, 2685–2695.
Thayer, M.J., Tapscott, S.J., Davis, R.L., Wright, W.E., Lassar, A.B., and Weintraub, H. (1989). Positive autoregulation of the myogenic determination gene MyoD1. Cell 58, 241–248.
Venuti, J.M., Morris, J.H., Vivian, J.L., Olson, E.N., and Klein, W.H. (1995). Myogenin is required for late but not early aspects of myogenesis during mouse development. J. Cell Biol. 128, 563–576.
Weintraub, H. (1993). The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75, 1241–1244.
Wright, W.E., Sassoon, D.A., and Lin, V.K. (1989). Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56, 607–617.
Wu, S.Y., and Chiang, C.M. (2007). The double bromodomain containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 282, 13141–13145.
Yang, Z., Yik, J.H., Chen, R., He, N., Jang, M.K., Ozato, K., and Zhou, Q. (2005). Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19, 535–545.
Zhang, H.J., Li, W.J., Gu, Y.Y., Li, S.Y., An, G.S., Ni, J.H., and Jia, H.T. (2010). p14ARF interacts with E2F factors to form p14ARFE2F/partner-DNA complexes repressing E2F-dependent transcription. J. Cell Biochem. 109, 693–701.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Du, C., **, YQ., Qi, JJ. et al. Effects of myogenin on expression of late muscle genes through MyoD-dependent chromatin remodeling ability of myogenin. Mol Cells 34, 133–142 (2012). https://doi.org/10.1007/s10059-012-2286-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-012-2286-1