Abstract
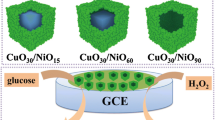
A multi-shelled NiO hollow sphere was synthesized by a facile glucose-mediated hydrothermal route. The carbonaceous microsphere was utilized as a sacrificial template for the formation of multi-shells. All the shells were formed by a single pyrolysis step. The multi-shelled hollow sphere can provide enhanced active surface area and additional reactive sites, which facilitate faster ion intercalation and deintercalation, and play key roles in electrochromic devices and sensing application, including glucose sensing. Herein, we employed the as-synthesized material for electrochromic devices. As expected, NiO multi-shelled hollow microspheres exhibit a superior transmission modulation (ΔT = 47%) and yield a coloration efficiency of ~ 85.3 cm2 C−1, which is ~ 2.37 times higher than that of NiO microflakes which was synthesized in the absence of glucose. The geometry of the self-supported multi-shelled architecture ensures that the active faradaic sites can be in intimate contact with the electrolyte to enhance ionic diffusion. The observed colored and bleached switching time of multi-shelled hollow sphere is 6.7 s and 2.7 s respectively. A quasi-solid-state electrochromic device is also displayed with the aid of gel electrolyte with reversible color change from dark brown to transparent. Furthermore, the multi-shelled NiO displayed excellent catalytic activity towards non-enzymatic glucose sensing with a high sensitivity of 1646 ± 5 μA cm−2 mM−1 over a linear range of 2 μM–2.6 mM with a lowest detection limit of 1.5 ± 0.2 μM. Analysis on blood serum as a real biological sample reflects the practicability of the fabricated sensor. Develo** hollow structured multi-shelled materials based on metal oxides will pave the way to design advanced electrode materials for electrochromic smart windows and non-enzymatic glucose sensors.

Bifunctional application of nickel oxide hollow microspheres in electrochromism and glucose sensing









Similar content being viewed by others
References
Granqvist CG (1995) Handbook of inorganic electrochromic materials, 1st edn. Elsevier, Amsterdam, The Netherlands
Spinner NS, Palmieri A, Beauregard N, Zhang L, Campanella J, Mustain WE (2015) Influence of conductivity on the capacity retention of NiO anodes in Li-ion batteries. J Power Sources 276:46–53
Mao D, Yao J, Lai X, Yang M, Du J, Wang D (2011) Hierarchically mesoporous hematite microspheres and their enhanced formaldehyde-sensing properties. Small 7(5):578–582
Cheng MY, Ye YS, Chiu TM, Pan CJ, Hwang BJ (2014) Size effect of nickel oxide for lithium ion battery anode. J Power Sources 253:27–34
Yue GH, Zhao YC, Wang CG, Zhang XX, Zhang XQ, **e QS (2015) Flower-like nickel oxide nanocomposites anode materials for excellent performance lithium-ion batteries. Electrochim Acta 152:315–322
Liu H, Wang G, Liu J, Qiao S, Ahn H (2011) Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J Mater Chem 21(9):3046–3052
Zhang Z, Gao Q, Gao H, Shi Z, Wu J, Zhi M, Hong Z (2016) Nickel oxide aerogel for high performance supercapacitor electrodes. RSC Adv 6(113):112620–112624
Vijayakumar S, Nagamuthu S, Muralidharan G (2013) Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl Mater Interfaces 5(6):2188–2196
Sohail I, Hussain Z, Khan AN, Yaqoob K (2017) Synthesis and characterization of electrodeposited NiO thin film on electrode grade carbon plate for supercapacitor applications. Mater Res Express 4(11):116412
Chen Y, Wang Y, Sun P, Yang P, Du L, Mai W (2015) Nickel oxide nanoflake-based bifunctional glass electrodes with superior cyclic stability for energy storage and electrochromic applications. J Mater Chem A 3(41):20614–20618
Lin F, Nordlund D, Weng TC, Moore RG, Gillaspie DT, Dillon AC, Richards RM, Engtrakul C (2013) Hole do** in Al-containing nickel oxide materials to improve electrochromic performance. ACS Appl Mater Interfaces 5(2):301–309
Liu Q, Chen Q, Zhang Q, **ao Y, Zhong X, Dong G, Delplancke-Ogletree MP, Terryn H, Baert K, Reniers F, Diao X (2018) In situ electrochromic efficiency of a nickel oxide thin film: origin of electrochemical process and electrochromic degradation. J Mater Chem C 6(3):646–653
Dalavi DS, Devan RS, Patil RS, Ma YR, Kang MG, Kim JH, Patil PS (2013) Electrochromic properties of dandelion flower like nickel oxide thin films. J Mater Chem A 1(4):1035–1039
Lin F, Nordlund D, Weng TC, Sokaras D, Jones KM, Reed RB, Gillaspie DT, Weir DGJ, Moore RG, Dillon AC, Richards RM, Engtrakul C (2013) Origin of electrochromism in high-performing nanocomposite nickel oxide. ACS Appl Mater Interfaces 5(9):3643–3649
Zhang C, Qian L, Zhang K, Yuan S, **ao J, Wang S (2015) Hierarchical porous Ni/NiO core–shells with superior conductivity for electrochemical pseudo-capacitors and glucose sensors. J Mater Chem A 3(19):10519–10525
Raza MH, Movlaee K, Wu Y, El-Refaei SM, Karg M, Leonardi SG, Neri G, Pinna N (2019) Tuning the NiO thin film morphology on carbon nanotubes by atomic layer deposition for enzyme-free glucose sensing. ChemElectroChem 6(2):383–392
Mishra S, Yogi P, Sagdeo PR, Kumar R (2018) Mesoporous nickel oxide (NiO) nanopetals for ultrasensitive glucose sensing. Nanoscale Res Lett 13(1):16
Soomro RA, Ibupoto ZH, Sirajuddin AMI, Willander M (2015) Controlled synthesis and electrochemical application of skein-shaped NiO nanostructures. J Solid State Electrochem 19(3):913–922
Yi W, Yang D, Chen H, Liu P, Tan J, Li H (2014) A highly sensitive nonenzymatic glucose sensor based on nickel oxide–carbon nanotube hybrid nanobelts. J Solid State Electrochem 18(4):899–908
El-Refaei SM, Saleh MM, Awad MI (2014) Tolerance of glucose electrocatalytic oxidation on NiOx/MnOx/GC electrode to poisoning by halides. J Solid State Electrochem 18(1):5–12
Elahi MY, Heli H, Bathaie SZ, Mousavi MF (2007) Electrocatalytic oxidation of glucose at a Ni-curcumin modified glassy carbon electrode. J Solid State Electrochem 11:273–282
Jafarian M, Forouzandeh F, Danaee I, Gobal F, Mahjani MG (2009) Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. J Solid State Electrochem 13(8):1171–1179
Browne MP, Nolan H, Berner NC, Duesberg GS, Colavita PE, Lyons MEG (2016) Electrochromic nickel oxide films for smart window applications. Int J Electrochem Sci 11:6636–6647
Cai GF, Tu JP, Zhang J, Mai YJ, Lu Y, Gu CD, Wang XL (2012) An efficient route to a porous NiO/reduced graphene oxide hybrid film with highly improved electrochromic properties. Nanoscale 4(18):5724–5730
Uplane MM, Mujawar SH, Inamdar AI, Shinde PS, Sonavane AC, Patil PS (2007) Structural, optical and electrochromic properties of nickel oxide thin films grown from electrodeposited nickel sulphide. Appl Surf Sci 253(24):9365–9371
Zhao C, Du F, Wang J (2015) Flower-like nickel oxide micro/nanostructures: synthesis and enhanced electrochromic properties. RSC Adv 5(48):38706–38711
Sialvi MZ, Mortimer RJ, Wilcox GD, Teridi AM, Varley TS, Wijayantha KGU, Kirk CA (2013) Electrochromic and colorimetric properties of nickel(II) oxide thin films prepared by aerosol-assisted chemical vapor deposition. ACS Appl Mater Interfaces 5(12):5675–5682
Zhou Y, Ni X, Ren Z, Ma J, Xu J, Chen X (2017) A flower-like NiO–SnO2 nanocomposite and its non-enzymatic catalysis of glucose. RSC Adv 7(71):45177–45184
Liu S, Yu B, Zhang T (2013) A novel non-enzymatic glucose sensor based on NiO hollow spheres. Electrochim Acta 102:104–107
Zhang WD, Chen J, Jiang LC, Yu YX, Zhang JQ (2010) A highly sensitive nonenzymatic glucose sensor based on NiO-modified multi-walled carbon nanotubes. Microchim Acta 168(3-4):259–265
Wang G, Lu X, Zhai T, Ling Y, Wang H, Tong Y, Li Y (2010) Free-standing nickel oxide nanoflake arrays: synthesis and application for highly sensitive non-enzymatic glucose sensors. Nanoscale 4:3123–3127
Zhang Y, Wang Y, Jia J, Wang J (2012) Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sensors Actuators B Chem 171-172:580–587
Li C, Liu Y, Li L, Du Z, Xu S, Zhang M, Yin X, Wang T (2008) A novel amperometric biosensor based on NiO hollow nanospheres for biosensing glucose. Talanta 77(1):455–459
Ci S, Huang T, Wen Z, Cui S, Mao S, Steeber DA, Chen J (2014) Nickel oxide hollow microsphere for non-enzyme glucose detection. Biosens Bioelectron 54:251–257
**ao H, Yao S, Liu H, Qu F, Zhang X, Wu X (2016) NiO nanosheet assembles for supercapacitor electrode materials. Prog Nat Sci: Mater Int 26(3):271–275
Liu N, Li J, Ma W, Liu W, Shi Y, Tao J, Zhang X, Su J, Li L, Gao Y (2014) Ultrathin and lightweight 3D free-standing Ni@NiO nanowire membrane electrode for a supercapacitor with excellent capacitance retention at high rates. ACS Appl Mater Interfaces 6(16):13627–13634
Zorkipli NNM, Kaus NHM, Mohamad AA (2016) Synthesis of NiO nanoparticles through sol-gel method. Procedia Chem 19:626–631
Zhu FL, Meng YS (2013) Preparation and characterization of NiO nanowires via single-phase precipitation. Adv Mater Res 668:331–334
Cruz-Ortiz BR, Garcia-Lobato MA, Larios-Durán ER, Múzquiz-Ramos EM, Ballesteros-Pacheco JC (2016) Potentiostatic electrodeposition of nanostructured NiO thin films for their application as electrocatalyst. J Electroanal Chem 772:38–45
Huang ML, Gu CD, Ge X, Wang XL, Tu JP (2014) NiO nanoflakes grown on porous graphene frameworks as advanced electrochemical pseudocapacitor materials. J Power Sources 259:98–105
Nkosi SS, Yalisi B, Motaung DE, Keartland J, Sideras-Haddad E, Forbes A, Mwakikunga BW (2013) Antiferromagnetic–paramagnetic state transition of NiO synthesized by pulsed laser deposition. Appl Surf Sci 265:860–864
Chai H, Chen X, Jia D, Bao S, Zhou W (2012) Flower-like NiO structures: controlled hydrothermal synthesis and electrochemical characteristic. Mater Res Bull 47(12):3947–3951
Jiang Y, Jia Z, Zhang W, Suo H (2013) In situ hydrothermal synthesis of nickel oxide nanostructures by thermal decomposition and its electrochemical property. J Inorg Organomet Polym Mater 23(4):1043–1047
Safa S, Hejazi R, Rabbani M, Azimirad R (2016) Hydrothermal synthesis of NiO nanostructures for photodegradation of 4-nitrophenol. Desalin Water Treat 57(46):21982–21989
Anandha Babu G, Ravi G, Mahalingam T, Kumaresavanji M, Hayakawa Y (2015) Influence of microwave power on the preparation of NiO nanoflakes for enhanced magnetic and supercapacitor applications. Dalton Trans 44(10):4485–4497
Wu H, Wang Y, Zheng C, Zhu J, Wu G, Li X (2016) Multi-shelled NiO hollow spheres: easy hydrothermal synthesis and lithium storage performances. J Alloys Compd 685:8–14
Chu L, Li M, Wan Z, Ding L, Song D, Dou S, Chen J, Wang Y (2014) Morphology control and fabrication of multi-shelled NiO spheres by tuning the pH value via a hydrothermal process. CrystEngComm 16(48):11096–11101
Purushothaman KK, Muralidharan G (2009) The effect of annealing temperature on the electrochromic properties of nanostructured NiO films. Sol Energy Mater Sol Cells 93(8):1195–1201
Lu Y, Liu L, Mandler D, Lee PS (2013) High switching speed and coloration efficiency of titanium-doped vanadium oxide thin film electrochromic devices. J Mater Chem C 1(44):7380–7386
Narayanan R, Dewan A, Chakraborty D (2018) Complimentary effects of annealing temperature on optimal tuning of functionalized carbon–V2O5 hybrid nanobelts for targeted dual applications in electrochromic and supercapacitor devices. RSC Adv 8(16):8596–8606
Cai G, Tu J, Zhou D, Li L, Zhang J, Wang X, Gu C (2014) Constructed TiO2/NiO core/shell nanorod array for efficient electrochromic application. J Phys Chem C 118(13):6690–6696
Zhang JH, Cai GF, Zhou D, Tang H, Wang XL, Gu CD, Tu JP (2014) Co-doped NiO nanoflake array films with enhanced electrochromic properties. J Mater Chem C 2(34):7013–7021
Tao L, Huo Z, Ding Y, Li Y, Dai S, Wang L, Zhu J, Pan X, Zhang B, Yao J, Nazeeruddin MK, Grätzel M (2015) High-efficiency and stable quasi-solid-state dye-sensitized solar cell based on low molecular mass organogelator electrolyte. J Mater Chem A 3(5):2344–2352
Prasad R, Ganesh V, Bhat BR (2016) Nickel-oxide multiwall carbon-nanotube/reduced graphene oxide a ternary composite for enzyme-free glucose sensing. RSC Adv 6(67):62491–62500
Chekin F, Bagheri S, Arof AK, Hamid SBA (2012) Preparation and characterization of Ni(II)/polyacrylonitrile and carbon nanotube composite modified electrode and application for carbohydrates electrocatalytic oxidation. J Solid State Electrochem 16(10):3245–3251
Marini S, Ben Mansour N, Hjiri M, Dhahri R, El Mir L, Espro C, Bonavita A, Galvagno S, Neri G, Leonardi SG (2018) Non-enzymatic glucose sensor based on nickel/carbon composite. Electroanalysis 30(4):727–733
Fan Y, Yang Z, Cao X, Liu P, Chen S, Cao Z (2014) Hierarchical macro-mesoporous Ni (OH) 2 for nonenzymatic electrochemical sensing of glucose. J Electrochem Soc 161(10):B201–B206
Zeng G, Li W, Ci S, Jia J, Wen Z (2016) Highly dispersed NiO nanoparticles decorating graphene nanosheets for non-enzymatic glucose sensor and biofuel cell. Sci Rep 6(1):36454
Mu Y, Jia D, He Y, Miao Y, Wu HL (2011) Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens Bioelectron 26(6):2948–2952
Soomro RA, Ibupoto ZH, Sirajuddin AMI, Willander M (2015) Electrochemical sensing of glucose based on novel hedgehog-like NiO nanostructures. Sensors Actuators B Chem 209:966–974
Shamsipur M, Najafi M, Hosseini MRM (2010) Highly improved electrooxidation of glucose at a nickel(II) oxide/multi-walled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 77(2):120–124
Ding Y, Wang Y, Su L, Zhang H, Lei Y (2010) Preparation and characterization of NiO–Ag nanofibers, NiO nanofibers, and porous Ag: towards the development of a highly sensitive and selective non-enzymatic glucose sensor. J Mater Chem 20(44):9918–9926
Wolfart F, Maciel A, Nagata N, Vidotti M (2013) Electrocatalytical properties presented by Cu/Ni alloy modified electrodes toward the oxidation of glucose. J Solid State Electrochem 17(5):1333–1338
Wang L, Tang Y, Wang L, Zhu H, Meng X, Chen Y, Sun Y, Yang XJ, Wan P (2015) Fast conversion of redox couple on Ni(OH)2/C nanocomposite electrode for high-performance nonenzymatic glucose sensor. J Solid State Electrochem 19(3):851–860
Ojani R, Raoof JB, Norouzi B (2011) Performance of glucose electrooxidation on Ni–Co composition dispersed on the poly(isonicotinic acid) (SDS) film. J Solid State Electrochem 15(6):1139–1147
Acknowledgments
We thank Dr. Shouvik Datta (IISER Pune) for valuable discussions.
Funding
RN acknowledges funding from the Department of Science and Technology, India, through the INSPIRE faculty scheme (DST/INSPIRE/04/2017/002761). AD and SH acknowledge the INSPIRE fellowship. We acknowledge the support from the Department of Science and Technology, India (Research Grant SR/NM/TP13/2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 814 kb)
Rights and permissions
About this article
Cite this article
Dewan, A., Haldar, S. & Narayanan, R. Multi-shelled NiO hollow microspheres as bifunctional materials for electrochromic smart window and non-enzymatic glucose sensor. J Solid State Electrochem 25, 821–830 (2021). https://doi.org/10.1007/s10008-020-04861-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04861-2