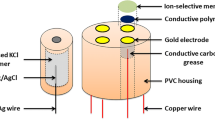
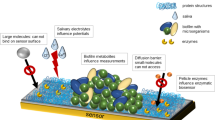
Abstract
Analysis of saliva is a potential diagnostic tool in the management of human diseases. Analysis of saliva in healthy individuals is vital to comparison in a diseased state. Salivary glandular secretion constantly bathes the teeth and oral mucosa. The presence of saliva is vital for healthy oral tissue. Positive correlation has been shown in salivary calcium and phosphate and oral health. We have developed a highly sensitive and selective impedimetric calcium sensor for its non-invasive determination in saliva. The sensor is based on 2-hydroxy-4-(2-oxo-1,2-diphenylethylidene)amino) benzoic acid ionophore; self-assembled monolayer (SAM) on gold electrode has been developed. The calcium sensor was constructed by SAM-Au formation of the compound developed by covalently attaching 4-aminothiophenol (ATP) to the ionophore molecule through amide bond formation between its amino group and the carboxylic group of the ionophore. Characterization of the SAM formation on the gold electrode was performed using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The modification of the gold electrode was confirmed by measuring the adhesion and surface morphology using contact angle (CA) measurements and SEM. EIS was used as the measuring technique; the sensor showed a linear analytical range (LR) of 5 × 10−12–1 × 10−6 mol L−1 and a limit of detection (LOD) of 3.6 × 10−12 mol L−1 calculated on the basis of 3σ/s and limit of quantification (LOQ) of 1.2 × 10−11 mol L−1. Moreover, the sensor was found to exhibit high selectivity for Ca2+selectivity over a variety of common interfering ions. The impedance behavior of the proposed calcium sensor has been modeled by an equivalent electrical circuit using a modified Randles model. The covalent immobilization of the ionophore into the modified gold electrode was manifested in its prolonged stability. The sensor was utilized for the determination of calcium concentration in real samples of human saliva; therefore, we believe it is suitable for point of care (POC).

Graphical abstract














Similar content being viewed by others
References
Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L (2013) Calcium in red blood cells—a perilous balance. Int J Mol Sci 14:9848–9872
Stewart TA, Yapa KTDS, Monteith GR (2015) Altered calcium signaling in cancer cells. Biochim Biophys Acta 1848(10 Pt B):2502–2511
Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y (2014) Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc Lond Ser B Biol Sci 369:20130097
Ross A C, Taylor C L, Yaktine A L, Del Valle H B (2011) Eds, IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press
Cashman KD (2002) Calcium intake, calcium bioavailability and bone health. Br J Nutr 87(Suppl. 2):S169–S177
Chojnowska S, Baran T, Wilinska I, Sienicka P, Cabaj-Wiater I, Knas M (2018) Human saliva as a diagnostic material. Adv Med Sci-Poland 63:185–191
Oyetola EO, Akomolafe R, Owotade FJ, Egunjobi S (2018) Physio-chemical analysis of unstimulated saliva of healthy Nigerian population. J Oral Biol 5:6
Garcia P T, Guimarães L N, Dias A A, Ulhoa C J, Coltro W K T (2018) Amperometric detection of salivary-amylase on screen-printed carbon electrodes as a simple and inexpensive alternative for point-of-care testing. Sens. Actuat B 258:342–348
Mohammad AJ, Ahad SA, Robert D, Simon DT (2016) Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 6:67–76
Liu J, Duan Y (2012) Saliva: a potential media for disease diagnostics and monitoring. Oral Oncol 48(7):569–577
Baumann T, Bereiter R, Lussi A, Carvalho TS (2017) The effect of different salivary calcium concentrations on the erosion protection conferred by the salivary pellicle. Sci Rep 7:12999
Yarat A, Altrufan E E, Akyuz S (2016) Calcium in saliva and impact on health, food and nutritional components in focus no. 10, calcium: chemistry, analysis, function and effects, Edited by Victor R. Preedy. The Royal Society of Chemistry
Zarei M (2017) Portable biosensing devices for point-of-care diagnostics: recent developments and applications. Trends Anal Chem 91:26–41
Abbas MN, Magar HS (2018) Highly sensitive and selective solid-contact calcium sensor based on Schiff base of benzil with 3-aminosalycilic acid covalently attached to polyacrylic acid amide for health care. J Solid State Electrochem 22:181–192
Burnett R W, Covington A K, Fogh-Andersen N, Külpmann W R, Lewenstam A, Maas A H J, Müller-Plathe O, VanKessel A L, Willem G Z (2000) Use of ion-selective electrodes for blood-electrolyte analysis. Recommendations for nomenclature, definitions and conventions, Clin. Chem. Lab Med 38:363–370
Bedlechowicz I, Sokalski T, Lewenstam A, Maj-Zurawska M (2005) Calcium ion-selective electrodes under galvanostatic current control. Sensors Actuators B 108:836–839
Wang SH, Chou TC, Liu C (2003) Development of a solid-state thick film calcium ion-selective electrode. Sensors Actuators B 96:709–716
Wang Y, Xu H, Yang X, Luo Z, Zhang J, Li G (2012) All-solid-state blood calcium sensors based on screen-printed poly(3,4-ethylenedioxythiophene) as the solid contact. Sensors Actuators B 173:630–635
Kumar A, Mittal SK (2004) PVC based dibenzo-18-crown-6 electrode for Ca (II) ions. Sensors Actuators B 99:340–343
Andreescu S, Sadik OA (2004) Trends and challenges in biochemical sensors for clinical and environmental monitoring. Pure Appl Chem 76:861–878
Sekretaryova AN, Eriksson M, Turner APF (2016) Bioelectrocatalytic systems for health applications. Biotechnol Adv 34(3):177–197
Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT (2015) Emerging technologies for next-generation point-of-care testing. Trends Biotechnol 33:692–705
Syedmoradi L, Daneshpour M, Alvandipour M, Gomez FA, Hajghassem H, Omidfar K (2017) Point of care testing: the impact of nanotechnology. Biosens Bioelectron 87:373–387
Gooding JJ, Mearns F, Yang W, Liu J (2003) Self-assembled monolayers into the 21st century: recent advances and applications. Electroanal. 15:81–96
Arya SK, Solanki PR, Datta M, Malhotra BD (2009) Recent advances in self-assembled monolayers based biomolecular electronic devices. Biosens Bioelectron 24(9):2810–2817
Vericat C, Vela ME, Benitez G, Carrob P, Salvarezza RC (2010) Self-assembled monolayers of thiols and dithiols on gold: new challenges for a well-known system. Chem Soc Rev 39(5):1805–1834
Schreiber F (2000) Structure and growth of self-assembling monolayers. Prog Surf Sci 65:151–256
Shiravand T, Azadbakht A (2017) Impedimetric biosensor based on bimetallic Ag Pt nanoparticle-decorated carbon nanotubes as highly conductive film surface. J Solid State Electrochem 21:1699–1711
Kochana J, Starzec K, Wieczorek M, Knihnicki P, Góra M, Rokicińska A, Kościelniak P, Kuśtrowski P (2019) Study on self-assembled monolayer of functionalized thiol on gold electrode forming capacitive sensor for chromium (VI) determination. J Solid State Electrochem 23:1463–1472
**ng YF, O’Shea SJ, Li SFY (2003) Electron transfer kinetics across a dodecanethiol monolayer self-assembled on gold. J Electroanal Chem 542:7–11
Jalal-Uddin M, Hossain MK, Hossain MI, Qarony W, Tayyaba S, Mia MNH, Pervez MF, Hossen S (2017) Modeling of self-assembled monolayers (SAMs) of octadecanethiol and hexadecanethiol on gold (Au) and silver (Ag). Results in Phys 7:2289–2295
Wesolowski M (1977) The decarboxylation of and thermal stability of p-aminosalicylic acid and its salts. Thermochim Acta 21:243–253
Cole B, Holt EM (1989) Alkali and alkaline earth complexation to derivatives of salicylic acid:[calcium (p-aminosalicylate)(acetate) (H2O)](H2O), magnesium (salicylate)2 (H2O)4, magnesium (p-aminosalicylate) 2(H2O)4, magnesium(2,6-pyridinedicarboxylate)-(H2O)3(H2O)2 and sodium (P-aminosalicylate) (H20)2. Inorg Chim Acta 160:195–203
Murugavel R, Korah R (2007) Structural diversity and supramolecular aggregation in calcium, strontium, and barium salicylates incorporating 1,10-phenanthroline and 4,4′-bipyridine: probing the softer side of group 2 metal ions with pyridinic ligands. Inorg Chem 46(26):11048–11062
Ukrainczyk M, Gredicak M, Jeric I, Kralj D (2012) Interactions of salicylic acid derivatives with calcite crystals. J Colloid Interface Sci 365:296–307
Patole J, Shingnapurkar D, Padhye S, Ratledge C (2006) Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Bioorg Med Chem Lett 16(6):1514–1517
Cesiulis H, Tsyntsaru N, Ramanavicius A, Ragoisha G (2016) The study of thin films by electrochemical impedance spectroscopy, I. Tiginyanu et al. (eds.), Nanostructures and thin films for multifunctional applications, nano science and technology, Springer International Publishing Switzerland
Richard P J, W. Ronald F, Abraham U (1998) Impedance spectroscopy of self-assembled monolayers on Au (111): sodium ferrocyanide charge transfer at modified electrodes. Langmuir 14:3011–3018
Itoa Y, Okuda-Shimazaki J, Tsugawa W, Loew N, Shitanda I, Lin C-E, La Belle J, Sod K (2019) Third generation impedimetric sensor employing direct electron transfer type glucose dehydrogenase. Biosens Bioelectron 129:189–119
Tounsi M, Ben Braiek M, Barhoumi H, Baraket A, Lee M, Zine N, Maaref A, Errachid A, (2016) A novel EIS field effect structures coated with TESUD-PPy-PVC-dibromoaza [7] helicene matrix for potassium ions detection, Mater. Sci. Eng. C. C 61: 608–615
Kumar GM, **ao F, Ilanchezhiyan P, Yuldashev S, Kang TW (2016) Enhanced photoelectrical performance of chemically processed SnS2 nanoplates. RSC Adv 6:99631
Kaur A, Kaur S, Sharma M, Kaur I (2019) Self-assembled monolayers of 3-hydroxy-N-(5-mercapto-1,3,4-thiadiazol-2-yl) benzamide (HMTB): a platform for impedimetric sensing of Co (II). J Electroanal Chem 833:221–230
Groß A, Sakong S (2019) Modelling the electric double layer at electrode/electrolyte interfaces. Curr Opin Electrochem 14:1–6
Lvova L, Kirsanov D, Di Natale C, Legin A (eds) (2013) Multisensor systems for chemical analysis: materials and sensors. CRC Press, Tailor & Francis, New York, p 74
Kim M, Iezzi R Jr., Shim B S , David C. Martin D C, (2019) Impedimetric biosensors for detecting vascular endothelial growth factor (VEGF) based on poly(3,4-ethylene dioxythiophene) (PEDOT)/gold nanoparticle (Au NP) composites, Front Chem 7:234
T. Erdey-Grúz, Kinetics of electrode processes (1972), Wiley New York
Finklea HO, Sinder DA, Fedyk J, Sabatani E, Gafni Y, Rubinstein I (1993) Characterization of octadecanethiol-coated gold electrodes as microarray electrodes by cyclic voltammetry and ac impedance spectroscopy. Langmuir 9:3660–3366
Oztekin Y, Ramanaviciene A, Ramanavicius A (2011) Electrochemical glutathione sensor based on electrochemically deposited poly-m-aminophenol. Electroanal. 23:701–709
Dzyadevich SV, Arhipova VA, Soldatkin AP, El’skaya AV, Shulga A A (1998) Glucose sensitive conductometric biosensor with additional Nafion membrane: reduction of influence of buffer capacity on the sensor response and extension of its dynamic range. Anal. Chim. Acta 374:11–18
Ganesh V, Kumar PS, Kumar S, Lakshminarayanan V (2006) Self-assembled monolayers (SAMs) of alkoxycyanobiphenyl thiols on gold—a study of electron transfer reaction using cyclic voltammetry and electrochemical impedance spectroscopy. J Colloid Interface Sci 296(1):195–203
Singh AK (2007) Calcium (II)-selective potentiometric sensor based on α furildioxime as neutral carrier. Sensors Actuators B 123:429–436
Lindfors T, Ivaska A (2001) Calcium-selective electrode based on polyaniline functionalize with bis[4-(1,1,3,3-tetramethylbutyl) phenyl] phosphate. Anal Chim Acta 437:171–182
Bedlechowicz-Śliwakowska I, Lingenfelter P, Sokalski T, Lewenstam A, Maj-Żurawska M (2006) Ion-selective electrode for measuring low Ca2+ concentrations in the presence of high K+, Na+ and Mg2+ background. Anal Bioanal Chem 385(8):1477–1482
Michalska A, Konopka A, Maj-Zurawska M (2003) All-solid-state calcium solvent polymeric membrane electrode for low-level concentration measurements. Anal Chem 75:141–144
Moore EW (1970) Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J Clin Invest 49(2):318–334
Israa MD, Sulafa KE (2018) Saliva and oral health. Int J Adv Res Biol Sci 5:1–45
Neyraud E, Dransfield E (2004) Relating ionization of calcium chloride in saliva to bitterness perception. Physiol Behav 81:505–510
Kumbhojkar SV, Kale AD, Kumbhojkar VR, Desai KM (2019) Salivary calcium as a diagnostic tool for screening of osteoporosis in postmenopausal women. J Oral Maxillofac Pathol 23(2):192–197
Abramova N, Vico JM, Soley J, Ocana C, Bratov A (2016) Solid contact ion sensor with conducting polymer layer copolymerized with the ion-selective membrane for determination of calcium in blood serum. Anal Chim Acta 943:50–57
Oliveira MDL, Melo CP, Oliva G, Andrade CAS (2011) Development of impedimetric optical calcium biosensor by using modified gold electrode with porcine S100A12 protein. Colloids Surf B Biointerfaces: Bio interfaces 82:365–370
Ankireddy SR, Kim J (2018) Highly selective and sensitive detection of calcium (II) ions in human serum using novel fluorescent carbon dots. Sensors Actuators B 255:3425–3433
Bi X, Wong WL, Ji W, Agarwal A, Balasubramanian N, Yang KL (2008) Development of electrochemical calcium sensors by using silicon nanowires modified with phosphotyrosine. Biosens Bioelectron 23(10):1442–1448
Akhtar MH, Hussain KK, Gurudatt NG, Shim YB (2017) Detection of Ca2+-induced acetylcholine released from leukemic T-cells using an amperometric microfluidic sensor. Biosens Bioelectron 98:364–370
Asif MH, Nur O, Willander M, Danielsson B (2009) Selective calcium ion detection with functionalized ZnO nanorods-extended gate MOSFET. Biosens Bioelectron 24(11):3379–3382
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Novelty
1. A newly synthesized Schiff base of benzil and p-aminosalicylic acid was used as novel calcium ionophore in an impedimetric sensor for the first time.
2. The SAM of ionophore developed on gold electrode using 4-aminothiophenol showed highly sensitive and selective impedimetric response to calcium ions over other interferent ions.
Electronic supplementary material
ESM 1
(DOC 403 kb)
Rights and permissions
About this article
Cite this article
Magar, H.S., Abbas, M.N., Ali, M.B. et al. Picomolar-sensitive impedimetric sensor for salivary calcium analysis at POC based on SAM of Schiff base–modified gold electrode. J Solid State Electrochem 24, 723–737 (2020). https://doi.org/10.1007/s10008-020-04500-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04500-w