Abstract
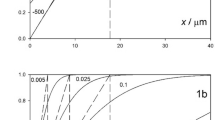
Simulations and experiments are reported which investigate the size of a macro disc electrode necessary to quantitatively show the chronoamperometric or voltammetric behaviour predicted by the Cottrell equation or the Randles-Sevcik equation on the basis of exclusive one-dimensional diffusional mass transport. For experimental time scales of several seconds, the contribution of radial diffusion is seen to be measurable even for electrodes of millimetres in radius. Recommendations on the size of macro electrodes for quantitative study are given and should exceed 4 mm radius in aqueous solution.



Similar content being viewed by others
Notes
The Arrhenius relation is ln D = constant − E aR−1 T −1; hence, the slope of the linear fit shown in Fig. 2b provides the activation energy for the diffusion of [Fe(CN)6]4− in the electrolyte upon multiplication by −R.
References
Amatore CA, Deakin MR, Wightman M (1986) Electrochemical kinetics at microelectrodes Part 1. Quasi-reversible electron transfer at cylinders. J Electroanal Chem Interfacial Electrochem 206:23–36. doi:10.1016/0022-0728(86)90253-6
Amatore C, Deakin MR, Wightman RM (1987) Electrochemical kinetics at microelectrodes: Part IV. Electrochemistry in media of low ionic strength. J Electroanal Chem Interfacial Electrochem 225:49–63. doi:10.1016/0022-0728(87)80004-9
Bond AM, Oldham KB, Zoski CG (1988) Theory of electrochemical processes at an inlaid disc microelectrode under steady-state conditions. J Electroanal Chem Interfacial Electrochem 245:71–104. doi:10.1016/0022-0728(88)80060-3
Bond AM (1994) Past, present and future contributions of microelectrodes to analytical studies employing voltammetric detection. A review. Analyst 119:1R–21R. doi:10.1039/AN994190001R
Scholz F (2009) Electroanalytical methods: guide to experiments and applications. Springer Science & Business Media
Montenegro MI, Montenegro I, Queirós MA, Daschbach JL (1991) Microelectrodes: theory and applications: theory and applications. Springer Science & Business Media
Mayrhofer KJJ, Strmcnik D, Blizanac BB et al (2008) Measurement of oxygen reduction activities via the rotating disc electrode method: from Pt model surfaces to carbon-supported high surface area catalysts. Electrochim Acta 53:3181–3188. doi:10.1016/j.electacta.2007.11.057
Concepcion JJ, Binstead RA, Alibabaei L, Meyer TJ (2013) Application of the rotating ring-disc-electrode technique to water oxidation by surface-bound molecular catalysts. Inorg Chem 52:10744–10746. doi:10.1021/ic402240t
Simonov AN, Kemppinen P, Pozo-Gonzalo C et al (2014) Aggregation of a Dibenzo[b, def]chrysene based organic photovoltaic material in solution. J Phys Chem B 118:6839–6849. doi:10.1021/jp501220v
Nicholson RS, Shain I (1964) Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal Chem 36:706–723. doi:10.1021/ac60210a007
Nicholson RS, Shain I (1965) Theory of stationary electrode polarography for a chemical reaction coupled between two charge transfers. Anal Chem 37:178–190. doi:10.1021/ac60221a002
Cottrell FG (1902) Z Für Phys Chem 42:385
Compton RG, Banks CE (2011) Understanding Voltammetry, 2nd ed. World Scientific
Fick A (1855) Ueber diffusion. Ann Phys 170:59–86. doi:10.1002/andp.18551700105
Fick A (1855) V. On liquid diffusion. Philos Mag Ser 4(10):30–39. doi:10.1080/14786445508641925
Morris GP, Simonov AN, Mashkina EA et al (2013) A comparison of fully automated methods of data analysis and computer assisted heuristic methods in an electrode kinetic study of the pathologically variable [Fe(CN)6]3–/4– process by AC voltammetry. Anal Chem 85:11780–11787. doi:10.1021/ac4022105
Bentley CL, Bond AM, Hollenkamp AF et al (2014) Applications of convolution voltammetry in electroanalytical chemistry. Anal Chem 86:2073–2081. doi:10.1021/ac4036422
Simonov AN, Morris GP, Mashkina EA et al (2014) Inappropriate Use of the quasi-reversible electrode kinetic model in simulation-experiment comparisons of voltammetric processes that approach the reversible limit. Anal Chem 86:8408–8417. doi:10.1021/ac5019952
Norouzi P, Ganjali MR, Daneshgar P, Mohammadi A (2007) Fast Fourier transform continuous cyclic voltammetry development as a highly sensitive detection system for ultra trace monitoring of thiamine. Anal Lett 40:547–559. doi:10.1080/00032710600964874
Ebrahimi B, Shojaosadati SA, Daneshgar P et al (2011) Performance evaluation of fast Fourier-transform continuous cyclic-voltammetry pesticide biosensor. Anal Chim Acta 687:168–176. doi:10.1016/j.aca.2010.12.005
Amatore C, Pebay C, Thouin L et al (2010) Difference between ultramicroelectrodes and microelectrodes: influence of natural convection. Anal Chem 82:6933–6939. doi:10.1021/ac101210r
Amatore C, Klymenko OV, Svir I (2012) Importance of correct prediction of initial concentrations in voltammetric scans: contrasting roles of thermodynamics, kinetics, and natural convection. Anal Chem 84:2792–2798. doi:10.1021/ac203188b
Barnes AS, Streeter I, Compton RG (2008) On the use of digital staircase ramps for linear sweep voltammetry at microdisc electrodes: large step potentials significantly broaden and shift voltammetric peaks. J Electroanal Chem 623:129–133. doi:10.1016/j.jelechem.2008.06.022
Ward KR, Compton RG (2014) Quantifying the apparent “Catalytic” effect of porous electrode surfaces. J Electroanal Chem 724:43–47. doi:10.1016/j.jelechem.2014.04.009
Ward KR, Gara M, Lawrence NS et al (2013) Nanoparticle modified electrodes can show an apparent increase in electrode kinetics due solely to altered surface geometry: the effective electrochemical rate constant for non-flat and non-uniform electrode surfaces. J Electroanal Chem 695:1–9. doi:10.1016/j.jelechem.2013.02.012
Compton RG, Laborda E, Ward KR (2013) Understanding voltammetry: simulation of electrode processes. Imperial College Press, London
Eloul S, Compton RG (2014) Shielding of a microdisc electrode surrounded by an adsorbing surface. Chem Electro Chem 1:917–924. doi:10.1002/celc.201400005
Konopka SJ, McDuffie B (1970) Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal Chem 42:1741–1746. doi:10.1021/ac50160a042
Lide DR (2008) CRC handbook of Chemistry and Physics, 89th ed. Taylor & Francis Group
Shoup D, Szabo A (1982) Chronoamperometric current at finite disk electrodes. J Electroanal Chem Interfacial Electrochem 140:237–245. doi:10.1016/0022-0728(82)85171-1
Heinze J (1981) Diffusion processes at finite (micro) disk electrodes solved by digital simulation. J Electroanal Chem Interfacial Electrochem 124:73–86. doi:10.1016/S0022-0728(81)80285-9
Heinze J, Storzbach M (1991) Digital Simulation of Mass Transport to Ultramicroelectrodes. Conference Proceeding NATO Advanced Study Inst on Microelectrodes: Theory and Applications
Britz D, Oldham KB, Østerby O (2009) Strategies for dam** the oscillations of the alternating direction implicit method of simulation of diffusion-limited chronoamperometry at disk electrodes. Electrochim Acta 54:4822–4828. doi:10.1016/j.electacta.2009.03.087
Britz D, Østerby O, Strutwolf J (2012) Minimum grid digital simulation of chronoamperometry at a disk electrode. Electrochim Acta 78:365–376. doi:10.1016/j.electacta.2012.06.009
Klymenko OV, Evans RG, Hardacre C et al (2004) Double potential step chronoamperometry at microdisk electrodes: simulating the case of unequal diffusion coefficients. J Electroanal Chem 571:211–221. doi:10.1016/j.jelechem.2004.05.012
**ong L, Aldous L, Henstridge MC, Compton RG (2012) Investigation of the optimal transient times for chronoamperometric analysis of diffusion coefficients and concentrations in non-aqueous solvents and ionic liquids. Anal Methods 4:371. doi:10.1039/c1ay05667k
Paddon CA, Bhatti FL, Donohoe TJ, Compton RG (2006) Cryo-electrochemistry in tetrahydrofuran: the electrochemical reduction of a phenyl thioether: [(3-{[trans-4-(Methoxymethoxy)cyclohexyl]oxy}propyl)thio]benzene. J Electroanal Chem 589:187–194. doi:10.1016/j.jelechem.2006.02.010
Acknowledgments
KT was supported by a Marie Curie Intra European Fellowship (Grant Agreement no. 327706) within the 7th European Community Framework Programme. SE and RGC acknowledge funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 320403.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper submitted for the ‘Fletcher Festschrift’ issue of Journal of Solid State Electrochemistry in admiration of an outstanding scientist and much valued electrochemistry colleague.
Rights and permissions
About this article
Cite this article
Ngamchuea, K., Eloul, S., Tschulik, K. et al. Planar diffusion to macro disc electrodes—what electrode size is required for the Cottrell and Randles-Sevcik equations to apply quantitatively?. J Solid State Electrochem 18, 3251–3257 (2014). https://doi.org/10.1007/s10008-014-2664-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2664-z