Abstract
The Fbw7–Skp1 complex is an essential component in the formation and development of the mammalian cardiovascular system; the complex interaction is mediated through binding of Skp1 C-terminal peptide (qGlu-peptide) to the F-box domain of Fbw7. By visually examining the crystal structure, we identified a typical cation ···π···π stacking system at the complex interface, which is formed by the Trp1159 residue of qGlu-peptide with the Lys2299 and His2359 residues of Fbw7 F-box domain. Both hybrid quantum mechanics/molecular mechanics (QM/MM) analysis of the real domain–peptide complex and electron-correlation ab initio calculation of the stacking system model suggested that the cation···π···π plays an important role in stabilizing the complex; substitution of peptide Trp1159 residue with aromatic Phe and Tyr would not cause a considerable effect on the configuration and energetics of cation···π···π stacking system, whereas His substitution seems to largely destabilize the system. Subsequently, the qGlu-peptide was stripped from the full-length Skp1 protein to define a so-called self-inhibitory peptide, which may rebind to the domain–peptide complex interface and thus disrupt the complex interaction. Fluorescence polarization (FP) assays revealed that the Trp1159Phe and Trp1159Tyr variants have a comparable or higher affinity (K d = 41 and 62 μM) than the wild-type qGlu-peptide (K d = 56 μM), while the Trp1159His mutation would largely impair the binding potency of qGlu-peptide to Fbw7 F-box domain (K d = 280 μM), confirming that the cation···π···π confers both affinity and specificity to the domain–peptide recognition, which can be reshaped by rational molecular design of the nonbonded interaction system.

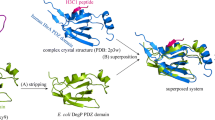
Stereoview of the complex structure of Fbw7 with Skp1 (PDB: 2ovp), where the Trp1159 residue of Skp1 qGlu-peptide can form a cation···π···π stacking system with the Lys2299 and His2359 residues of Fbw7 F-box domain.






Similar content being viewed by others
References
Welcker M, Clurman BE (2008) FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8:83–93
Tetzlaff MT, Yu W, Li M, Zhang P, Finegold M, Mahon K, Harper JW, Schwartz RJ, Elledge SJ (2004) Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci U S A. 101:3338–3345
Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ (1996) SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86:263–274
Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP (2007) Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell 26:131–143
Petsalaki E, Russell RB (2008) Peptide-mediated interactions in biological systems: new discoveries and applications. Curr Opin Biotechnol 19:344–350
London N, Raveh B, Movshovitz-Attias D, Schueler-Furman O (2010) Can self-inhibitory peptides be derived from the interfaces of globular protein–protein interactions? Proteins 78:3140–3149
Word JM, Lovell SC, Richardson JS, Richardson DC (1999) Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. J Mol Biol. 285:1735–1747
Gordon JC, Myers JB, Folta T, Shoja V, Heath LS, Onufriev A (2005) H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 33:W368–W371
Senn HM, Thiel W (2009) QM/MM methods for biomolecular systems. Angew Chem Int Ed Engl 48:1198–1229
Chung LW, Sameera WM, Ramozzi R, Page AJ, Hatanaka M, Petrova GP, Harris TV, Li X, Ke Z, Liu F, Li HB, Ding L, Morokuma K (2015) The ONIOM method and its applications. Chem Rev. 115:5678–5796
Lu Y, Shi T, Wang Y, Yang H, Yan X, Luo X, Jiang H, Zhu W (2009) Halogen bonding—a novel interaction for rational drug design? J Med Chem 52:2854–2862
Zhou P, Zou J, Tian F, Shang Z (2009) Fluorine bonding—how does it work in protein–ligand interactions? J Chem Inf Model. 49:2344–2355
Lu Y, Zou J, Fan J, Zhao W, Jiang Y, Yu Q (2009) Ab initio calculations on halogen-bonded complexes and comparison with density functional methods. J Comput Chem. 30:725–732
Mourik T (2008) Assessment of density functionals for intramolecular dispersion-rich interactions. J Chem Theory Comput. 4:1610–1619
Benitex Y, Baranger AM (2011) Control of the stability of a protein–RNA complex by the position of fluorine in a base analogue. J Am Chem Soc. 133:3687–3689
Duan Y, Wu C, Chowdhury S, Lee MC, **ong G, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang J, Kollman P (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 24:1999–2012
Frisch MJ, Trucks GW, Schlegel HB (2009) Gaussian 09. Gaussian Inc., Wallingford, CT
Tian F, Lv Y, Zhou P, Yang L (2011) Characterization of PDZ domain-peptide interactions using an integrated protocol of QM/MM, PB/SA, and CFEA analyses. J Comput Aided Mol Des. 25:947–958
Rocchia W, Alexov E, Honig B (2001) Extending the applicability of the nonlinear. Poisson–Boltzmann equation: multiple dielectric constants and multivalent ions. J Phys Chem 105:6507–6514
Kollman PA, Massova I, Reyes C, Kuhn B, Huo SH, Chong L, Lee M, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, Cheatham TE (2000) Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res. 33:889–897
Sanner MF, Olson AJ, Spehner JC (1996) Reduced surface: an efficient way to compute molecular surfaces. Biopolymers 38:305–320
Dunning TH (1989) The atoms boron through neon and hydrogen. J Chem Phys. 90:1007–1023
Chesnut DB, Moseley RW (1969) The geometries of molecular complexes: an extended Hückel approach. Theor Chim Acta 13:230–248
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Krivov GG, Shapovalov MV, Dunbrack RL (2009) Improved prediction of protein side-chain conformations with SCWRL4. Proteins 77:778–795
An Y, Bloom JW, Wheeler SE (2015) Quantifying the π-stacking interactions in nitroarene binding sites of proteins. J Phys Chem B 119:14441–14450
Zhuo ZH, Sun YZ, ** PN, Li FY, Zhang YL, Wang HL (2016) Selective targeting of MAPK family kinases JNK over p38 by rationally designed peptides as potential therapeutics for neurological disorders and epilepsy. Mol BioSyst. 12:2532–2540
Chao Yang, Shilei Zhang, ** He, Congcong Wang, Jian Huang, Peng Zhou, (2015) Self-Binding Peptides: Folding or Binding?. Journal of Chemical Information and Modeling 55 (2):329–342
Chao Yang, Shilei Zhang, Zhengya Bai, Shasha Hou, Di Wu, Jian Huang, Peng Zhou, (2016) A two-step binding mechanism for the self-binding peptide recognition of target domains. Mol. BioSyst. 12 (4):1201–1213
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81500188).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary materials
Supplementary material
1 (PDB 160 kb)
Supplementary material
2 (PDB 4 kb)
Supplementary material
3 (PDB 4 kb)
Supplementary material
4 (PDB 5 kb)
Supplementary material
5 (PDB 4 kb)
Rights and permissions
About this article
Cite this article
Zhou, J., Wang, YS. Rational redesign of a cation···π···π stacking at cardiovascular Fbw7–Skp1 complex interface and its application for deriving self-inhibitory peptides to disrupt the complex interaction. J Mol Model 23, 296 (2017). https://doi.org/10.1007/s00894-017-3456-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3456-z