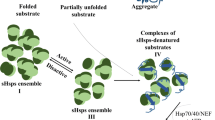
Abstract
Citrate synthase (CS) is often used in chaperone assays since this thermosensitive enzyme aggregates at moderately increased temperatures. Small heat shock proteins (sHsps) are molecular chaperones specialized in preventing the aggregation of other proteins, termed substrate proteins, under conditions of transient heat stress. To investigate the mechanism whereby sHsps bind to and stabilize a substrate protein, we here used peptide array screening covering the sequence of porcine CS (P00889). Strong binding of sHsps was detected to a peptide corresponding to the most N-terminal α-helix in CS (amino acids Leu13 to Gln27). The N-terminal α-helices in the CS dimer intertwine with the C-terminus in the other subunit and together form a stem-like structure which is protruding from the CS dimer. This stem-like structure is absent in thermostable forms of CS from thermophilic archaebacteria like Pyrococcus furiosus and Sulfolobus solfatacarium. These data therefore suggest that thermostabilization of thermosensitive CS by sHsps is achieved by stabilization of the C- and N-terminae in the protruding thermosensitive softspot, which is absent in thermostable forms of the CS dimer.




Similar content being viewed by others
References
Arnott MA, Michael RA, Thompson CR, Hough DW, Danson MJ (2000) Thermostability and thermoactivity of citrate synthases from the thermophilic and hyperthermophilic archaea, Thermoplasma acidophilum and Pyrococcus furiosus. J Mol Biol 304:657–668
Arrigo AP (2005) In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J Cell Biochem 94:241–246
Basha E, Lee GJ, Breci LA, Hausrath AC, Buan NR, Giese KC, Vierling E (2004) The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. J Biol Chem 279:7566–7575
Blennow A, Surin BP, Ehring H, McLennan NF, Spangfort MD (1995) Isolation and biochemical characterization of highly purified Escherichia coli molecular chaperone Cpn60 (GroEL) by affinity chromatography and urea-induced monomerization. Biochim Biophys Acta 1252:69–78
Boros S, Kamps B, Wunderink L, de Bruijn W, de Jong WW, Boelens WC (2004) Transglutaminase catalyzes differential crosslinking of small heat shock proteins and amyloid-beta. FEBS Lett 576:57–62
Buchner J, Ehrnsperger M, Gaestel M, Walke S (1998a) Purification and characterization of small heat shock proteins. Methods Enzymol 290:339–349
Buchner J, Grallert H, Jakob U (1998b) Analysis of chaperone function using citrate synthase as non-native substrate protein. Methods Enzymol 290:323–338
Clark JI, Muchowski PJ (2000) Small heat-shock proteins and their potential role in human disease. Curr Opin Struct Biol 10:52–59
Ehrnsperger M, Graber S, Gaestel M, Buchner J (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 16:221–229
Frank R (1992) Spot-synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 48:9217–9232
Frank R (2002) The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports—principles and applications. J Immunol Methods 267:13–26
Gustavsson N, Kokke BP, Anzelius B, Boelens WC, Sundby C (2001) Substitution of conserved methionines by leucines in chloroplast small heat shock protein results in loss of redox-response but retained chaperone-like activity. Protein Sci 10:1785–1793
Han MJ, Park SJ, Park TJ, Lee SY (2004) Roles and applications of small heat shock proteins in the production of recombinant proteins in Escherichia coli. Biotechnol Bioeng 88:426–436
Han MJ, Lee JW, Lee SY (2005) Enhanced proteome profiling by inhibiting proteolysis with small heat shock proteins. J Proteome Res 4:2429–2434
Harndahl U, Hall RB, Osteryoung KW, Vierling E, Bornman JF, Sundby C (1999) The chloroplast small heat shock protein undergoes oxidation-dependent conformational changes and may protect plants from oxidative stress. Cell Stress Chaperones 4:129–138
Harndahl U, Kokke BP, Gustavsson N, Linse S, Berggren K, Tjerneld F, Boelens WC, Sundby C (2001) The chaperone-like activity of a small heat shock protein is lost after sulfoxidation of conserved methionines in a surface-exposed amphipathic alpha-helix. Biochim Biophys Acta 1545:227–237
Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J (1999) Hsp26: a temperature-regulated chaperone. EMBO J 18:6744–6751
Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12:842–846
Horwitz J (1992) Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA 89:10449–10453
Horwitz J (2003) Alpha-crystallin. Exp Eye Res 76:145–153
Hultschig C, Frank R (2004) Multiplexed sorting of libraries on libraries: a novel method for empirical protein design by affinity-driven phage enrichment on synthetic peptide arrays. Mol Divers 8:231–245
Jakob U, Gaestel M, Engel K, Buchner J (1993) Small heat shock proteins are molecular chaperones. J Biol Chem 268:1517–1520
Jiao W, Li P, Zhang J, Zhang H, Chang Z (2005) Small heat-shock proteins function in the insoluble protein complex. Biochem Biophys Res Commun 335:227–231
Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW (2003) The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones 8:53–61
Kim KK, Kim R, Kim SH (1998) Crystal structure of a small heat-shock protein. Nature 394:595–599
Koch J, Mahler M, Bluthner M, Dubel S (2002) Analysis of protein interactions with peptides synthesized on membranes. In: Golemis E (ed) Protein–protein interactions. CSHL Press, New York, pp 569–583
Lee GJ, Roseman AM, Saibil HR, Vierling E (1997) A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J 16:659–671
Lethanh H, Neubauer P, Hoffmann F (2005) The small heat-shock proteins IbpA and IbpB reduce the stress load of recombinant Escherichia coli and delay degradation of inclusion bodies. Microb Cell Fact 4:6
Matuszewska M, Kuczynska Wisnik D, Laskowska E, Liberek K (2005) The small heat shock protein IbpA of Escherichia coli cooperates with IbpB in stabilization of thermally aggregated proteins in a disaggregation competent state. J Biol Chem 280:12292–12298
Nordberg Karlsson E, Crennell SJ, Higgins C, Nawaz S, Yeoh L, Hough DW, Danson MJ (2003) Citrate synthase from Thermus aquaticus: a thermostable bacterial enzyme with a five-membered inter-subunit ionic network. Extremophiles 7:9–16
Remington S, Wiegand G, Huber R (1982) Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol 158:111–152
Russell RJ, Hough DW, Danson MJ, Taylor GL (1994) The crystal tructure of citrate synthase from the thermophilic archaeon, Thermoplasma acidophilum. Structure 2:1157–1167
Russell RJ, Ferguson JM, Hough DW, Danson MJ, Taylor GL (1997) The crystal structure of citrate synthase from the hyperthermophilic archaeon Pyrococcus furiosus at 1.9 A resolution. Biochemistry 36:9983–9994
Sharma KK, Kumar RS, Kumar GS, Quinn PT (2000) Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem 275:3767–3771
Stamler R, Kappe G, Boelens W, Slingsby C (2005) Wrap** the alpha-crystallin domain fold in a chaperone assembly. J Mol Biol 353:68–79
Van Montfort R, Slingsby C, Vierling E (2001) Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem 59:105–156
van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E (2001) Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol 8:1025–1030
Waters ER, Vierling E (1999) The diversification of plant cytosolic small heat shock proteins preceded the divergence of mosses. Mol Biol Evol 16:127–139
Acknowledgment
This study was supported by the Carl Tryggers Research Foundation and Magnus Bergvalls Stiftelse.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian.
Rights and permissions
About this article
Cite this article
Åhrman, E., Gustavsson, N., Hultschig, C. et al. Small heat shock proteins prevent aggregation of citrate synthase and bind to the N-terminal region which is absent in thermostable forms of citrate synthase. Extremophiles 11, 659–666 (2007). https://doi.org/10.1007/s00792-007-0080-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0080-3