Abstract
Objectives
Τhis study aims at determining the ability of cone beam computed tomography (CBCT) to visualize critical-size defects (CSD) created at rat calvaria and filled with 75/25 w/w nano-hydroxyapatite/chitosan (nHAp/CS) scaffolds, prior to their histological investigation.
Materials and methods
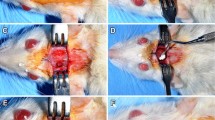
Thirty adult Sprague Dawley rats, 15 males and 15 females, were used. Two CSD, 5 mm in diameter, were bilaterally trephined in the parietal bone. The right CSD was filled with nHAp/CS scaffold, while the left CSD remained empty, as the control group. Two female rats died post-operatively. Rats were euthanized at 2, 4, and 8 weeks post-surgery. Twenty-eight specimens (15 × 2 × 10 mm) were resected—containing both CSDs—and then scanned using a NewTom VGi CBCT imaging unit (Verona, Italy). The manufacturer’s software trace region profile tool (NNT v6.2, Verona, Italy) was used in selected axial slices. The greyscale value (in VGiHU) and the traced/selected region of interest (ROI, in mm2) of those areas were automatically calculated. Subsequently, all specimens were histologically examined.
Results
An increased VGiHU (P = 0.000), was observed in the experimental group relative to the control group. The ROI of CSD (in mm2) was significantly reduced (P = 0.001) from the fourth to the eighth week in both groups. No statistically significant difference between male and female rats (P = 0.188) was observed with respect to VGiHU.
Conclusions
The nHAp/CS scaffolds are easily visualized using a particular high-resolution CBCT device.
Clinical relevance
Both the CBCT measurements and also the histological results suggest that the nHAp/CS scaffold presence contributes to new bone formation in rat calvarial CSD.







Similar content being viewed by others
References
Gomes PS, Fernandes MH (2011) Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim 45:14–24
Bosch C, Melsen B, Vargervik K (1998) Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg 9:310–316
Donos N, Lang NP, Karoussis IK, Bosshardt D, Tonetti M, Kostopoulos L (2004) Effect of GBR in combination with deproteinized bovine bone mineral and/or enamel matrix proteins on the healing of critical-size defects. Clin Oral Implants Res 15:101–111
Donos N, Dereka X, Mardas N (2015) Experimental models for guided bone regeneration in healthy and medically compromised conditions. Periodontol 68:99–121
Ghiacci G, Graiani G, Ravanetti F, Lumetti S, Manfredi E, Galli C, Cacchioli A, Macaluso GM, Sala R (2016) “Over-inlay” block graft and differential morphometry: a novel block graft model to study bone regeneration and host-to-graft interfaces in rats. J Periodontal Implant Sci 46(4):220–223
Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M et al (2005) Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J ClinPeriodontol 32:966–972
Buser D, Hoffman B, Bernard JP, Lussi A, Mettler D, Schenk RK (1998) Evaluation of filling materials in membrane protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implants Res 9:137–150
Burchardt H (1987) Biology of bone transplantation. Orthop Clin North Am 18:187–196
Kurz LT, Garfin SR, Booth REJ (1989) Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine 14:1324–1331
Shalash MA, Rahman HA, Azim AA, Neemat AH, Hawary HE, Nasry SA (2013) Evaluation of horizontal ridge augmentation using beta tricalcium phosphate and demineralized bone matrix: a comparative study. J Clin Exp Dent 5:253–259
Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: current concepts and future directions. BMC Med 9:66
Moore WR, Graves SE, Bain GI (2001) Synthetic bone graft substitutes. ANZ J Surg 71:354–361
Navarro M, Michiardi A, Castano O, Planell JA (2008) Biomaterials in orthopaedics. J R Soc Interface 5:1137–1158
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431
Bonucci E (2000) Basic composition and structure of bone. In: An YH, Draughn RA (eds) Mechanical testing of bone and the bone-implant interface. CRC Press LLC Boca Raton, USA, pp 3–21
Tsiourvas D, Sapalidis A, Papadopoulos T (2016) Hydroxyapatite/chitosan-based porous three-dimensional scaffolds with complex geometries. Mater Today 7:59–66
O’Brien FJ (2011) Biomaterials and scaffolds for tissue engineering. Mater Today 14:88–95
Vlierberghe SV, Dubruel P, Schacht E (2011) Biopolymer-based hydrogels as Scaffolds for tissue engineering applications: a review. Biomacromolecules 2:1387–1408
Kong L, Gao Y, Lu G, Gong Y, Zhao N, Zhang X (2006) A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur Polym J 42:3171–3179
Kashiwazaki H, Kishiya Y, Matsuda A, Yamaguchi K, Iizuka T, Tanaka J, Inoue N (2009) Fabrication of porous chitosan/hydroxyapatite nanocomposites: their mechanical and biological properties. BioMed Mater Eng 19:133–140
Thein-Han WW, Misra RDK (2009) Three-dimensional chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. JOM J Miner Met Mater Soc 61:41–44
Hench LL (1998) Bioceramics. J Am Ceram Soc 81:1705–1728
Blaker JJ, Gough JE, Maquet V, Notingher I, Boccaccini AR (2003) In vitro evaluation of novel bioactive composites based on bioglass-filledpolylactide foams for bone tissue engineering scaffolds. J Biomed Mater Res 67A:1401–1411
Castro F, Kuhn S, Jensen K, Ferreira A, Rocha F, Vicente A et al (2013) Continuous-flow precipitation of hydroxyapatite in ultrasonic microsystems. Chem Eng J 215:979–987
Cui W, Li X, **e C, Chen J, Zou J, Zhou S, Weng J (2010) Controllable growth of hydroxyapatite on electrospun poly(dl-lactide) fibers grafted with chitosan as potential tissue engineering scaffolds. Polymer 51:2320–2328
Di Martino A, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983–5990
Tigli RS, Karakecili A, Gumusderelioglu M (2007) In vitro characterization of chitosan scaffolds: influence of composition and deacetylation degree. J Mater Sci Mater Med 18:1665–1674
Nakamura T, Shirakata Y, Shinohara Y, Miron RJ, Hasegawa-Nakamura K, Fujioka-Kobayashi M, Noguchi K (2017) Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin Oral Investig 21(9):2671–2679
Zhang H, Mao X, Du Z, Jiang W, Han X, Zhao D et al (2016) Three dimensional printed macroporouspolylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci Technol Adv Mater 17:136–148
Ito K, Gomi Y, Sato S, Arai Y, Shinoda K (2001) Clinical application of a new compact CT system to assess 3-D images for the preoperative treatment planning of implants in the posterior mandible. A case report. Clin Oral Implants Res 12:539–542
Pauwels R, Beinsberger J, Collaert B, Theodorakou C, Rogers J, Walker A, Cockmartin L, Bosmans H, Jacobs R, Bogaerts R, Horner K, SEDENTEXCT Project Consortium (2012) Effective dose range for dental cone beam computed tomography scanners. Eur J Radiol 81:267–271
Mah P, Reeves TE, McDavid WD (2010) Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofac Radiol 39:323–335
Lagravère MO, Carey J, Toogood RW, Major PW (2008) Three-dimensional accuracy of measurements made with software on cone-beam computed tomography images. Am J Orthod Dentofac Orthop 134:112–116
Aranyarachkul P, Caruso J, Gantes B, Schulz E, Riggs M, Dus I, Yamada JM, Crigger M (2005) Bone density assessments of dental implant sites. 2. Quantitative cone-beam computerized tomography. Int J Oral Maxillofac Implants 20:416–424
Naitoh M, Hirukawa A, Katsumata A, Ariji E (2009) Evaluation of voxel values in mandibular cancellous bone: relationship between cone-beam computed tomography and multislice helical computed tomography. Clin Oral Implants Res 20:503–506
Naitoh M, Hirukawa A, Katsumata A, Ariji E (2010) Prospective study to estimate mandibular cancellous bone density using large-volume cone-beam computed tomography. Clin Oral Implants Res 21:1309–1313
Nomura Y, Watanabe H, Honda E, Kurabayashi T (2010) Reliability of voxel values from cone-beam computed tomography for dental use in evaluating bone mineral density. Clin Oral Implants Res 21:558–562
Lagravère MO, Fang Y, Carey J, Toogood RW, Packota GV, Major PW (2006) Density conversion factor determined using a cone-beam computed tomography unit NewTom QR-DVT 9000. DentomaxillofacRadiol 35:407–409
Campos MJ, de Souza TS, MotaJúnior SL, Fraga MR, Vitral RW (2014) Bone mineral density in cone beam computed tomography: only a few shades of gray. World J Radiol 28;6(8):607–612
ICRP (2011) Radiation protection: cone-beam CT for dental and maxillofacial radiology. Evidence based guidelines [online]. Report prepared by the SEDENTEXCT project. March 2011. Available from: http://www.sedentexct.eu/files/radiation_protection_172.pdf
Ponder SN, Benavides E, Kapila S, Hatch NE (2013) Quantification of external root resorption by low-vs high- resolution cone-beam computed tomography and periapical radiography: a volumetric and linear analysis. Am J Orthod Dentofac Orthop 143:77–91
Van Dessel J, Nicolielo LFP, Huang Y, Coudyzer W, Salmon B, Lambrichts I, Jacobs R (2017) Accuracy and reliability of different cone beam computed tomography (CBCT) devices for structural analysis of alveolar bone in comparison with multislice CT and micro-CT. Eur J Oral Implantol 10(1):95–105
Liang X, Zhang Z, Gu J, Wang Z, Vandenberghe B, Jacobs R, Yang J, Ma G, Ling H, Ma X (2017) Comparison of micro-CT and cone beam CT on the feasibility of assessing trabecular structures in mandibular condyle. Dentomaxillofac Radiol 46:20160435
Kulah K, Gulsahi A, Kamburoglu K, Geneci F, Ocak M, Celik HH, Ozen T (2018) Evaluation of maxillary trabecular microstructure as an indicator of implant stability by using 2 cone beam computed tomography systems and micro-computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. https://doi.org/10.1016/j.oooo.2018.11.014
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Tsiourvas D, Tsetsekou A, Kammenou MI, Boukos N (2011) Controlling the formation of hydroxyapatite nanorods with dendrimers. J Am Ceram Soc 94:2023–2029
Kuo YC, Tsai YT (2010) Inverted colloidal crystal scaffolds for uniform cartilage regeneration. Biomacromolecules 11:731–739
Kim RW, Kim JH, Moon SY (2016) Effect of hydroxyapatite on critical-sized defect. Maxillofac Plast Reconstr Surg 38:1–6
Gao R, Watson M, Callon KE, Tuari D, Dray M, Naot D et al (2018) Local application of lactoferrin promotes bone regeneration in a rat critical-sized calvarial defect model as demonstrated by micro-CT and histological analysis. J Tissue Eng Regen Med 12:620–626
Johari B, Ahmadzadehzarajabad M, Azami M, Kazemi M, Soleimani M, Kargozar S, Hajighasemlou S, Farajollahi MM, Samadikuchaksaraei A (2016) Repair of rat critical size calvarial defect using osteoblast-like and umbilical vein endothelial cells seeded in gelatin/hydroxyapatite scaffolds. J Biomed Mater Res 104:1770–1778
Oryan A, Alidadi S, Bigham-Sadegh A, Meimandi-Parizi A (2017) Chitosan/gelatin/platelet gel enriched by o combination of hydroxyapatite and beta-tricalcium phosphate in healing of radial bone defect model in rat. Int J Biol Macromol 101:630–637
He Y, Dong Y, Cui F, Chen X, Lin R (2015) Ectopic osteogenesis and scaffold biodegradation of nano-hydroxyapatite-chitosan in a rat model. PLoS One 10:1–15
Groppo MF, Caria PH, Freire AR, Figueroba SR, Ribeiro-Neto WA, Bretas RES (2017) The effect of a hydroxyapatite impregnated PCL membrane in rat subcritical calvarial bone defects. Arch Oral Biol 82:209–215
Lohmann P, Willuweit A, Neffe AT, Geisler S, Gebauer TP, Beer S, Coenen HH, Fischer H, Hermanns-Sachweh B, Lendlein A, Shah NJ, Kiessling F, Langen KJ (2017) Bone regeneration induced by a 3D architectured hydrogel in a rat critical-size calvarial defect. Biomaterials 113:158–169
de Santana WM, de Sousa DN, Ferreira VM, Duarte WR (2016) Simvastatin and biphasic calcium phosphate affects bone formation in critical-sized rat calvarial defects. Acta Cir Bras 31:300–307
Schulz KF (1996) Randomised trials, human nature, and reporting guidelines. Lancet 348:596–598
Pauwels R, Jacobs R, Singer SR, Mupparapu M (2015) CBCT-based bone quality assessment: are Hounsfield units applicable? DentomaxillofacRadiol 44:1–16
Cankaya AB, Erdem MA, Isler SC, Demircan S, Soluk M, Kasapoglu C, Oral CK (2011) Use of cone-beam computerized tomography for evaluation of bisphosphonate-associated osteonecrosis of the jaws in an experimental rat model. Int J Med Sci 8(8):667–672
Chatzipetros E, Christopoulos P, Donta C, Tosios KI, Tsiambas E, Tsiourvas D, Kalogirou EM, Tsiklakis K (2018) Application of nano-hydroxyapatite/chitosan scaffolds on rat calvarial critical-sized defects: a pilot study. Med Oral Patol Oral Cir Bucal 23(5):625–632
Acknowledgments
Hyperbranched poly (ethyleneimine) was kindly donated by BASF Hellas S.A.
Funding
This research is co-financed by Greece and the European Union (European Social Fund—ESF) through the Operational Programme “Human Resources Development, Education and Lifelong Learning” in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research” (MIS-5000432), implemented by the State Scholarships Foundation (ΙΚΥ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
All animal experimental protocols and procedures were approved by the Directorate of Agricultural and Veterinary Policy with protocol number 1181/2-03-2017 and registration code EL 25 BIO 05, Athens, Greece.
Informed consent
Informed consent was not required in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chatzipetros, E., Yfanti, Z., Christopoulos, P. et al. Imaging of nano-hydroxyapatite/chitosan scaffolds using a cone beam computed tomography device on rat calvarial defects with histological verification. Clin Oral Invest 24, 437–446 (2020). https://doi.org/10.1007/s00784-019-02939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02939-4