Abstract
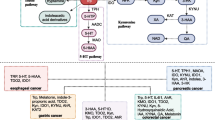
Amino acids not only play a vital role in the synthesis of biological molecules such as proteins in cancer malignant cells, they are also essential metabolites for immune cell activation and antitumor effects in the tumor microenvironment. The abnormal changes in amino acid metabolism are closely related to the occurrence and development of tumors and immunity. Intestinal microorganisms play an essential role in amino acid metabolism, and tryptophan and its intestinal microbial metabolites are typical representatives. However, it is known that the cyclic amino acid profile is affected by specific cancer types, so relevant studies mainly focus on one type of cancer and rarely study different cancer forms at the same time. The objective of this study was to examine the PFAA profile of five cancer patients and the characteristics of tryptophan intestinal microbial metabolites to determine whether there are general amino acid changes across tumors. Plasma samples were collected from esophageal (n = 53), lung (n = 73), colorectal (n = 94), gastric (n = 55), breast cancer (n = 25), and healthy control (HC) (n = 139) subjects. PFAA profile and tryptophan metabolites were measured, and their perioperative changes were examined using high-performance liquid chromatography. Univariate analysis revealed significant differences between cancer patients and HC. Furthermore, multivariate analysis discriminated cancer patients from HC. Regression diagnosis models were established for each cancer group using differential amino acids from univariate analysis. Receiver-operating characteristic analysis was applied to evaluate these diagnosis models. Finally, GABA, arginine, tryptophan, taurine, glutamic acid, and melatonin showed common alterations across all types of cancer patients. Metabolic pathway analysis shows that the most significant enrichment pathways were tryptophan, arginine, and proline metabolism. This study provides evidence that common alterations of the metabolites mentioned above suggest their role in the pathogenesis of each cancer patient. It was suggested that multivariate models based on PFAA profiles and tryptophan metabolites might be applicable in the screening of cancer patients.






Similar content being viewed by others
Data availability
The data that support the findings of the current study are available on reasonable request from the corresponding author. The data are not publicly available due to restrictions e.g. containing information that could compromise the privacy of research participants.
References
Abramjuk C et al (2009) Divergent effects of taurolidine as potential anti-neoplastic agent: Inhibition of bladder carcinoma cells in vitro and promotion of bladder tumor in vivo. Oncol Rep 22(2):409–414
Aceto N et al (2009) Taurolidine and oxidative stress: a rationale for local treatment of mesothelioma. Eur Respir J 34(6):1399–1407
Agrawal A et al (2016) Role of melatonin in the pathophysiology of cancer. J Chron DD 7:1–6
Ando S et al (2003) The significance of tumour markers as an indication for mediastinoscopy in non-small cell lung cancer. Respirology 8(2):163–167
Ashino H et al (2003) Novel function of ascorbic acid as an angiostatic factor. Angiogenesis 6:259–269
Bowles TL et al (2008) Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer 123(8):1950–1955
Braumann C et al (2009) Taurolidine reduces the tumor stimulating cytokine interleukin-1beta in patients with resectable gastrointestinal cancer: a multicentre prospective randomized trial. World J Surg Oncol 7(1):1–13
Brzozowska A et al (2017) Gamma-amino butyric acid (GABA) level as an overall survival risk factor in breast cancer. Ann Agric Environ Med 24(3):435–439
Burke L et al (2020) The Janus-like role of proline metabolism in cancer. Cell Death Discovery 6(1):104
Daigeler A et al (2008) Synergistic apoptotic effects of taurolidine and TRAIL on squamous carcinoma cells of the esophagus. Int J Oncol 32(6):1205–1220
Danaceau JP et al (2003) A liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. J Anal Toxicol 27(7):440–444
Dereziński P et al (2017) Amino acid profiles of serum and urine in search for prostate cancer biomarkers: a pilot study. Int J Med Sci 14(1):1
Dols MC et al (2006) Specific alterations in the serum amino acid profile of patients with lung cancer and head and neck cancer. Oncol 29(7):283–290
El Agouza I et al (2011) Taurine: a novel tumor marker for enhanced detection of breast cancer among female patients. Angiogenesis 14:321–330
El-Fattah A, Eslam E (2022) IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med 20(1):1–13
Grant RS (2018) Indoleamine 2, 3-Dioxygenase activity increases NAD+ production in IFN-γ–stimulated human primary mononuclear cells. Int J Tryptophan Res 11:1178646917751636
Grzywa TM et al (2020) Myeloid cell-derived arginase in cancer immune response. Front Immunol 11:938
Gu Y et al (2015) Perioperative dynamics and significance of amino acid profiles in patients with cancer. J Transl Med 13(1):1–14
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143(1):29–36
Heng B et al (2016) Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget 7(6):6506
Hoksch B et al (2009) Taurolidine in the prevention and therapy of lung metastases. Eur J Cardiothorac Surg 36(6):1058–1063
Hu L et al (2016) Identification of arginine and its “Downstream” molecules as potential markers of breast cancer. IUBMB Life 68(10):817–822
Huang S et al (2016) Novel personalized pathway-based metabolomics models reveal key metabolic pathways for breast cancer diagnosis. Genome Med 8(1):1–14
Iwan P, Stepniak J, Karbownik-Lewinska M (2021) Cumulative protective effect of melatonin and indole-3-propionic acid against KIO3—induced lipid peroxidation in porcine thyroid. Toxics 9(5):89
Lei X, Tie J (2019) Prediction of disease-related metabolites using bi-random walks. PLoS ONE 14(11):e0225380
Liu S, Madu CO, Lu Y (2018) The role of melatonin in cancer development. Oncomedicine 3(1):37–47
Löb S et al (2009) IDO1 and IDO2 are expressed in human tumors: levo-but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58(1):153–157
Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10(12):723–736
Mates JM et al (2012) Sulphur-containing non enzymatic antioxidants: therapeutic tools against cancer. Front Biosci (schol Ed) 4:722–748
Minuk GY et al (2007) Decreased hepatocyte membrane potential differences and GABAa-β3 expression in human hepatocellular carcinoma. Hepatology 45(3):735–745
Miyagi Y et al (2011) Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE 6(9):e24143
Munn DH, Mellor AL (2013) Indoleamine 2, 3 dioxygenase and metabolic control of immune responses. Trends Immunol 34(3):137–143
Ogiso H et al (2017) The inhibition of indoleamine 2, 3-dioxygenase accelerates early liver regeneration in mice after partial hepatectomy. Dig Dis Sci 62(9):2386–2396
Oruganty K et al (2020) Common biochemical properties of metabolic genes recurrently dysregulated in tumors. Cancer Metab 8:1–15
Phang JM et al (2015) Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care 18(1):71
Rath M et al (2014) Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol 5:532
Sánchez-Alcoholado L et al (2020) The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 12(6):1406
Sawicka MM et al (2022) Proline metabolism in malignant gliomas: a systematic literature review. Cancers 14(8):2030
Simińska E, Koba M (2016) Amino acid profiling as a method of discovering biomarkers for early diagnosis of cancer. Amino Acids 48(6):1339–1345
Stapleton PP et al (1998) Host defense—a role for the amino acid taurine? J Parenter Enter Nutr 22(1):42–48
Su X, Gao Y, Yang R (2022) Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells 11(15):2296
Sun N, Zhao X (2022) Argininosuccinate synthase 1, arginine deprivation therapy and cancer management. Front Pharmacol. https://doi.org/10.3389/fphar.2022.935553
Synakiewicz A, Stachowicz-Stencel T, Adamkiewicz-Drozynska E (2014) The role of arginine and the modified arginine deiminase enzyme ADI-PEG 20 in cancer therapy with special emphasis on Phase I/II clinical trials. Expert Opin Investig Drugs 23(11):1517–1529
Thapar R, Titus MA (2014) Recent advances in metabolic profiling and imaging of prostate cancer. Curr Metabolomics 2(1):53–69
Vissers YL et al (2005) Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am J Clin Nutr 81(5):1142–1146
Wan Y et al (2019) Indole: a privileged scaffold for the design of anti-cancer agents. Eur J Med Chem 183:111691
Watanabe M et al (2006) Gamma-aminobutyric acid GABA and cell proliferation, focus on cancer cells. Histol histopathol
Weems JM, Yost GS (2010) 3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem Res Toxicol 23(3):696–704
Weng T et al (2018) Recent discovery of indoleamine-2, 3-dioxygenase 1 inhibitors targeting cancer immunotherapy. Eur J Med Chem 143:656–669
Ye Z et al (2019) Role of IDO and TDO in cancers and related diseases and the therapeutic implications. J Cancer 10(12):2771
Yu J, Kim AK (2009) Effect of taurine on antioxidant enzyme system in B16F10 melanoma cells. Taurine 7. Springer, pp 491–499
Zhang J et al (2010) Suppression of hypoxia-inducible factor 1α (HIF-1α) by tirapazamine is dependent on eIF2α phosphorylation rather than the mTORC1/4E-BP1 pathway. PLoS ONE 5(11):e13910
Zhang J et al (2021) Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: a therapeutic target to control intestinal inflammation. Med Res Rev 41(2):1061–1088
Zhao Q et al (2014) Plasma and tissue free amino acid profiles and their concentration correlation in patients with lung cancer. Asia Pac J Clin Nutr 23(3):429–436
Acknowledgements
The authors acknowledge the support of Gulou Hospital and Southeast University, Nan**g, China.
Funding
This study was funded by the National Science Foundation of China (No. 82173575), the Fundamental Research Funds for the Central Universities (2242021k30014), and the Fundamental Research Funds for the Central Universities (2242021k30059).
Author information
Authors and Affiliations
Contributions
Ahad Hussain, First author, wrote the main manuscript text and statistical analysis Li **e. Equal contribution, method development and manuscript revision Guozhe Dengc. Experimental method development Xuejun Kang. Corresponding author, conceptualization, manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Handling editor: S. Broeer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hussain, A., **e, L., Deng, G. et al. Common alterations in plasma free amino acid profiles and gut microbiota-derived tryptophan metabolites of five types of cancer patients. Amino Acids 55, 1189–1200 (2023). https://doi.org/10.1007/s00726-023-03308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-023-03308-y