Abstract
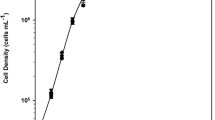
Growth of five aeroterrestrial green algal strains (Trebouxiophyceae) in response to changing water availabilities—caused by osmotic (ionic) and matric (desiccation) stresses—was investigated in comparison with a freshwater and a marine strain. All investigated algae displayed good growth under brackish conditions while four out of the five aeroterrestrial strains even grew well under full marine conditions (28–40 psu). The comparison between growth responses in liquid medium, on solid agarose, and on glass fiber filters at 100% air humidity indicated a broad growth tolerance of aeroterrestrial algae towards diminished water availability. While two aeroterrestrial strains even grew better on solid medium which mimics natural biofilm conditions, the aquatic strains showed significant growth inhibition under matric stress. Except Stichococcus sp., which contained the C6-polyol sorbitol, all other aeroterrestrial green algae investigated synthesized and accumulated the C5-polyol ribitol in response to osmotic stress. Using 13C NMR spectroscopy and HPLC, it could be verified that ribitol functions as an osmotically regulated organic solute. This is the first proof of ribitol in free-living aeroterrestrial green algae. The biochemical capability to synthesize polyols under environmental stress conditions seems to support algal life outside aquatic habitats.





Similar content being viewed by others
References
Armstrong RA, Smith SN (1994) The levels of ribitol, arabitol and mannitol in individual lobes of the lichen Parmelia conspersa. (Ehrh ex Ach) Ach. Environ Exp Bot 34:253–260
Bertsch A (1966) CO2-exchange and water relations in the aerophilic green alga Apatococcus lobatus. Planta 70:46–50
Bisson MA, Kirst GO (1995) Osmotic acclimation and turgor pressure regulation in algae. Naturwiss 82:461–471
Blanchard GF, Guarini JM, Richard P, Gros P, Mornet F (1996) Quantifying the short-term temperature effect on light-saturated photosynthesis of intertidal microphytobenthos. Mar Ecol Prog Ser 134:309–313
Brown LM, Hellebust JA (1978) Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can J Bot 56:676–679
Brown LM, Hellebust JA (1980) The contribution of organic solutes to osmotic balance in some green and eustigmatophyte algae. J Phycol 16(2):265–270
Brown AD, Simpson JR (1972) Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J Gen Microbiol 72:589–591
Brownell KH, Schneider RW (1985) Roles of matric and osmotic components of water potential and their interaction with temperature in the growth of Fusarium oxysporum in synthetic media and soil. Phytopath 75:53–57
Chang WS, van de Mortel M, Nielsen L, de Guzman GN, Li XH, Halverson LJ (2007) Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J Bacteriol 189:8290–8299
Cowan AK, Rose PD, Horne LG (1992) Dunaliella salina — a model system for studying the response of plant-cells to stress. J Exp Bot 43:1535–1547
Dewinder B, Matthijs HCP, Mur LR (1990) The effect of dehydration and ion stress on carbon-dioxide fixation in drought-tolerant phototrophic microorganisms. Fems Microb Ecol 74:33–38
Eggert A, Karsten U (2009) Low molecular weight carbohydrates in red algae — an ecophysiological and biochemical perspective. In: Seckbach J, Chapman, D, Weber A (eds) Cellular origins, life in extreme habitats and astrobiology — red algae in the genomics age. Springer, Berlin (in press)
Eggert A, Häubner N, Klausch S, Karsten U, Schumann R (2006) Quantification of algal biofilms colonising building materials: chlorophyll a measured by PAM-fluorometry as a biomass parameter. Biofouling 22:79–90
Eggert A, Nitschke U, West JA, Michalik D, Karsten U (2007) Acclimation of the intertidal red alga Bangiopsis subsimplex (Stylonematophyceae) to salinity changes. J Exp Mar Biol Ecol 343:176–186
Erdmann N, Hagemann M (2007) Salt acclimation of algae and cyanobacteria: a comparison. In: Rai LC, Gaur JP (eds) Algal adaptation to environmental stresses: physiological, biochemical and molecular mechanisms. Springer, Berlin, pp 322–360
Feige GB, Kremer BP (1980) Unusual carbohydrate pattern in Trentepohlia species. Phytochem 19:1844–1845
Fraymouth J (1928) The moisture relations of terrestrial algae. III. The respiration of certain lower plants, including terrestrial algae, with special reference to the influence of drought. Ann Bot 42:75–100
Gilmour DJ, Hipkins MF, Boney AD (1984) The effect of osmotic and ionic stress on the primary processes of photosynthesis in Dunaliella tertiolecta. J Exp Bot 35:18–27
Gray DW, Lewis LA, Cardon ZG (2007) Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant Cell Environ 30:1240–1255
Gustavs L, Schumann R, Eggert A, Karsten U (2009) In vivo growth fluorometry: accuracy and limits of microalgal growth rate measurements in ecophysiological investigations. Aquat Microb Ecol 55:95–104
Häubner N, Schumann R, Karsten U (2006) Aeroterrestrial microalgae growing in biofilms on facades — response to temperature and water stress. Microb Ecol 51:285–293
Hinton RH, Burge MLE, Hartman GC (1969) Sucrose interference in the assay of enzymes and proteins. Anal Biochem 29:248–256
Jacob A, Kirst GO, Wiencke C, Lehmann H (1991) Physiological responses of the antarctic green-alga Prasiola crispa ssp antarctica to salinity stress. J Plant Physiol 139:57–62
Karsten U, Thomas DN, Weykam G, Daniel C, Kirst GO (1991) A simple and rapid method for extraction and separation of low-molecular-weight carbohydrates from macroalgae using high-performance liquid-chromatography. Plant Physiol Biochem 29:373–378
Karsten U, West JA, Mostaert A, King R, Barrow K, Kirst GO (1992) Mannitol in the red algal genus Caloglossa (Harvey) J Agardh. J Plant Physiol 140:292–297
Karsten U, West JA, Zuccarello G, Kirst GO (1994) Physiological ecotypes in the marine red alga Bostrychia radicans (Ceramiales, Rhodophyta) from the east coast of the USA. J Phycol 30:174–182
Karsten U, Klimant I, Holst G (1996) A new in vivo fluorimetric technique to measure growth of adhering phototrophic microorganisms. Appl Environ Microbiol 62:237–243
Karsten U, Michalik D, Michalik M, West JA (2005) A new unusual low molecular weight carbohydrate in the red algal genus Hypoglossum (Delesseriaceae, Ceramiales) and its possible function as an osmolyte. Planta 222:319–326
Karsten U, Schumann R, Mostaert A (2007) Aeroterrestrial algae growing on man-made surfaces: what are the secrets of their ecological success? In: Seckbach J (ed) Algae and cyanobacteria growing in extreme environments. Springer, Berlin, pp 585–597
Kirst GO (1990) Salinity tolerance of eukaryotic algae. Annu Rev Plant Physiol Plant Mol Biol 41:21–53
Lange OL, Bilger W, Rimke S, Schreiber U (1989) Chlorophyll fluorescence of lichens containing green and blue-green-algae during hydration by water-vapor uptake and by addition of liquid water. Bot Acta 102:306–313
Lange OL, Meyer A, Budel B (1994) Net photosynthesis activation of a desiccated cyanobacterium without liquid water in high air humidity alone — experiments with Microcoleus sociatus isolated from a desert soil crust. Funct Ecol 8:52–57
Lu WD, Chi ZM, Su CD (2006) Identification of glycine betaine as compatible solute in Synechococcus sp. WH8102 and characterization of its N-methyltransferase genes involved in betaine synthesis. Arch Microbiol 186:495–506
Mackay MA, Norton RS, Borowitzka LJ (1984) Organic osmoregulatory solutes in cyanobacteria. J Gen Microbiol 130:2177–2191
Mudimu O (2008) Biodiversity of green algal biofilms on artificial hardsubstrates. Dissertation at the Georg-August University Göttingen, Germany
Nash TH, Lange OL (1988) Responses of lichens to salinity — concentration and time-course relationships and variability among Californian species. New Phytol 109:361–367
Ong BL, Lim M, Wee YC (1992) Effects of desiccation and illumination on photosynthesis and pigmentation of an edaphic population of Trentepohlia odorata (Chlorophyta). J Phycol 28:768–772
Ophir T, Gutnick DL (1994) A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl Environ Microbiol 60:740–745
Orcutt DM, Parker BC, Lusby WR (1986) Lipids in blue-green-algal mats (modern stromatolites) from Antarctic Oasis lakes. J Phycol 22:523–530
Ortega-Calvo JJ, Arino X, Hernandez-Marine M, Saiz-Jimenez C (1995) Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci Total Environ 167:329–341
Palmer RJ, Friedmann EI (1990) Water relations and photosynthesis in the cryptoendolithic microbial habitat of hot and cold deserts. Microb Ecol 19:111–118
Palmqvist K (2000) Carbon economy in lichens. New Phytol 148:11–36
Papendick RI, Campbell GS (1980) Theory and measurement of water potential. In: Parr JF, Gardner WR, Elliot LF (eds) Water potential relations in soil microbiology. Soil Science Society of America, Madison, WI, USA, pp 1–22
Potts M (1999) Mechanisms of desiccation tolerance in cyanobacteria. E J Phycol 34:319–328
Ramirez ML, Chulze SN, Magan N (2004) Impact of osmotic and matric water stress on germination, growth, mycelial water potentials and endogenous accumulation of sugars and sugar alcohols in Fusarium graminearum. Mycologia 96:470–478
Reed RH (1989) Osmotic adjustment and organic solute accumulation in Chaetomorpha capillaris. Brit Phycol J 24:21–37
Reed RH, Richardson DL, Warr SR, Stewart WD (1984) Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol 130:1–4
Richardson HS, Smith DC (1968) Lichen physiology.9. Carbohydrate movement from Trebouxia symbiont of Xanthoria aureola to fungus. New Phytol 67:469–486
Rindi F (2007) Diversity, distribution and ecology of green algae and cyanobacteria in urban habitats. In: Seckbach J (ed) Algae and cyanobacteria growing in extreme environments. Springer, Berlin, pp 619–638
Rindi F, McIvor L, Guiry MD (2004) The Prasiolales (Chlorophyta) of Atlantic Europe: An assessment based on morphological, molecular, and ecological data, including the characterization of Rosenvingiella radicans (Kutzing) comb. nov. J Phycol 40:977–997
Rindi F, Sherwood AR, Guiry MD (2005) Taxonomy and distribution of Trentepohlia and Printzina (Trentepohliales, Chlorophyta) in the Hawaiian Islands. Phycologia 44:270–284
Roberts MF (2006) Characterization of organic compatible solutes of halotolerant and halophilic microorganisms. In: Rainey F, Oren A (eds) Extremophiles (methods in microbiology). Elsevier, London, UK, pp 615–648
Roser DJ, Melick DR, Ling HU, Seppelt RD (1992) Polyol and sugar content of terrestrial plants from continental Antarctica. Ant Sci 4:413–420
Schloesser U (1994) SAG-Sammlung von Algenkulturen at the university of Goettingen — catalogue of strains (medium 5). Bot Acta 107:113–186
Setter TL, Greenway H (1979) Growth and osmoregulation of Chlorella emersonii in NaCl and neutral osmotica. Aust J Plant Physiol 6:47–60
Starr RC, Zeikus JA (1993) UTEX — the culture collection of algae at the university of Texas at Austin 1993 list of cultures. J Phycol 29:1–106
Tomaselli L, Lamenti G, Bosco M, Tiano P (2000) Biodiversity of photosynthetic micro-organisms dwelling on stone monuments. Intern Biodet Biodeg 46:251–258
Van Alstyne KL, Pelletreau KN, Rosario K (2003) The effects of salinity on dimethylsulfoniopropionate production in the green alga Ulva fenestrata Postels et Ruprecht (Chlorophyta). Bot Mar 46:350–356
van de Mortel M, Halverson LJ (2004) Cell envelope components contributing to biofilm growth and survival of Pseudomonas putida in low-water-content habitats. Mol Microbiol 52:735–750
van den Hoek C, Mann DG, Jahns HM (1995) Algae: an introduction to phycology. Cambridge University Press, UK
Warr SRC, Reed RH, Stewart WDP (1988) The compatibility of osmotica in cyanobacteria. Plant Cell Environ 11:137–142
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Acknowledgements
The authors thank Christiane Volkmann for technical support, as well as Prof. Thomas Friedl, University of Göttingen and Dr. Thomas Pröschold, Dunstaffnage Marine Laboratory, Oban for providing taxonomical information on the strains studied. Images b and d of Fig. 1 is in courtesy of C. Mudimu, Kiel. We gratefully appreciate financial support through the Deutsche Forschungsgemeinschaft (DFG KA 899/13-1/2 and DFG EG 151/1-2).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Cornelius Lütz on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Gustavs, L., Eggert, A., Michalik, D. et al. Physiological and biochemical responses of green microalgae from different habitats to osmotic and matric stress. Protoplasma 243, 3–14 (2010). https://doi.org/10.1007/s00709-009-0060-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0060-9